The role of probiotics, prebiotics, and postbiotics: cellular and molecular pathways activated on glial cells in Alzheimer's disease
- PMID: 40636703
- PMCID: PMC12237913
- DOI: 10.3389/fnins.2025.1598011
The role of probiotics, prebiotics, and postbiotics: cellular and molecular pathways activated on glial cells in Alzheimer's disease
Abstract
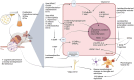
Supplementation with prebiotics and probiotics can modulate the intestinal microbiota, returning it to a more physiological state; therefore, they can be considered as a possible treatment in many prevalent conditions, including neurodegenerative diseases. Alzheimer's disease (AD) is the most common form of dementia, accounting for 60 to 70% of cases. The neuropathological features of AD include neuritic plaques (extracellular deposits of the beta-amyloid protein, Aβ), neurofibrillary tangles (resulting from hyperphosphorylation of the tau protein), a predominantly cholinergic synaptic decrease, and the presence of inflammatory markers, all these characteristics together trigger the neurodegenerative process and cognitive deterioration. The etiology of AD is multifactorial, however, in recent years evidence has been shown on the significant association between dysbiosis, neuroinflammation, and neurodegeneration. In the present review, we will discuss the role of gut microbiota in the pathogenesis of AD, as well as the underlying mechanisms that trigger the use of probiotics, prebiotics, and postbiotics in neuroinflammation. Our attention will focus on the cellular and molecular mechanisms triggered by astrocytes and microglia, cells involved in mediating neuroinflammation and neurodegeneration in AD.
Keywords: Alzheimer’s disease; glial cells; neuroinflammation; postbiotic; prebiotic; probiotic.
Copyright © 2025 Patricio-Martínez, Patricio, Macuil-Chapuli, Martínez-Juárez, Flores-Díaz, Cedillo-Ramírez and Limón.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Synbiotics, prebiotics and probiotics for solid organ transplant recipients.Cochrane Database Syst Rev. 2022 Sep 20;9(9):CD014804. doi: 10.1002/14651858.CD014804.pub2. Cochrane Database Syst Rev. 2022. PMID: 36126902 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Synbiotics, prebiotics and probiotics for people with chronic kidney disease.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD013631. doi: 10.1002/14651858.CD013631.pub2. Cochrane Database Syst Rev. 2023. PMID: 37870148 Free PMC article.
-
The Gut Microbiota Modulates Neuroinflammation in Alzheimer's Disease: Elucidating Crucial Factors and Mechanistic Underpinnings.CNS Neurosci Ther. 2024 Oct;30(10):e70091. doi: 10.1111/cns.70091. CNS Neurosci Ther. 2024. PMID: 39460538 Free PMC article. Review.
References
-
- Alberdi E., Sánchez-Gómez M. V., Cavaliere F., Pérez-Samartín A., Zugaza J. L., Trullas R., et al. (2010). Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47, 264–272. doi: 10.1016/j.ceca.2009.12.010, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

