Oxidation of Alcohols to Carboxylates with N2O Catalyzed by Ruthenium(II)-CNC Complexes
- PMID: 40636731
- PMCID: PMC12235671
- DOI: 10.1021/acscatal.5c02021
Oxidation of Alcohols to Carboxylates with N2O Catalyzed by Ruthenium(II)-CNC Complexes
Abstract
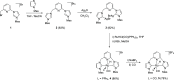
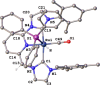
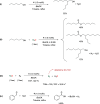
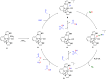
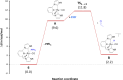
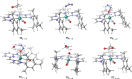
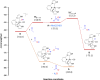
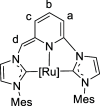
Air-stable ruthenium-(II) complexes based on a picoline-derived CNC pincer ligand, [RuH-(CNC)-(CO)L]X (L = PPh3, X = Br; L = CO, X = BF4), were found to catalyze under basic conditions the oxidation with N2O of a series of alcohols to carboxylates. Both [RuH-(CNC)-(CO)L]X complexes react readily with strong bases (tBuOK or KHMDS), giving rise to a Ru-(II) complex containing a deprotonated CNC* ligand (when L = PPh3) or a Ru(0)-CNC derivative (for L = CO). Furthermore, the mechanism of the catalytic reaction has been elucidated through density functional theory (DFT) calculations. The catalytic cycle has been shown to proceed through an outer-sphere mechanism comprising four key transformations, which involve Ru-(II) intermediates based on the deprotonated CNC* ligand: (i) alkoxide dehydrogenation to yield a Ru-(II) hydride complex and an aldehyde molecule, (ii) N2O insertion into the ruthenium-hydride bond to yield a hydroxy ruthenium species and N2, (iii) nucleophilic attack of the hydroxo ligand in the Ru-OH complex to the intermediate aldehyde, and (iv) dehydrogenation of the formed alcoholate to regenerate the catalytically active Ru-(II) hydride and produce the carboxylate product.
Keywords: alcohols; carboxylates; nitrous oxide; ruthenium complexes; transfer hydrogenation.
© 2025 The Authors. Published by American Chemical Society.
Figures


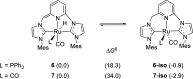


Similar articles
-
Theoretical Study on Photocatalytic CO2 Reduction to Formate by a Ruthenium CNC Pincer Complex.J Phys Chem A. 2025 Aug 26. doi: 10.1021/acs.jpca.5c04348. Online ahead of print. J Phys Chem A. 2025. PMID: 40859116
-
Productive Homogeneous Hydrogenation of Fatty Esters with Carboxylate SNS Ruthenium Catalysts.Chemistry. 2025 Aug 7;31(44):e202501898. doi: 10.1002/chem.202501898. Epub 2025 Jul 16. Chemistry. 2025. PMID: 40626952 Free PMC article.
-
Pyridine-Quinoline and Biquinoline-Based Ruthenium p-Cymene Complexes as Efficient Catalysts for Transfer Hydrogenation Studies: Synthesis and Structural Characterization.Molecules. 2025 Jul 11;30(14):2945. doi: 10.3390/molecules30142945. Molecules. 2025. PMID: 40733211 Free PMC article.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.Health Technol Assess. 2007 Nov;11(45):iii-iv, ix-221. doi: 10.3310/hta11450. Health Technol Assess. 2007. PMID: 17999840
-
A health-based recommended occupational exposure limit for nitrous oxide using experimental animal data based on a systematic review and dose-response analysis.Environ Res. 2021 Oct;201:111575. doi: 10.1016/j.envres.2021.111575. Epub 2021 Jun 23. Environ Res. 2021. PMID: 34174259
References
-
- Hansen J., Sato M.. Greenhouse Gas Growth Rates. Proc. Natl. Acad. Sci. U. S. A. 2004;101:16109–16114. doi: 10.1073/pnas.0406982101. - DOI - PMC - PubMed
- Rodhe H.. A Comparison of the Contribution of Various Gases to the Greenhouse Effect. Science. 1990;248:1217–1219. doi: 10.1126/science.248.4960.1217. - DOI - PubMed
- Montzka S. A., Dlugokencky E. J., Butler J. H.. Non-CO2 Greenhouse Gases and Climate Change. Nature. 2011;476:43–50. doi: 10.1038/nature10322. - DOI - PubMed
-
- Prather M. J.. Time Scales in Atmospheric Chemistry: Coupled Perturbations to N2O, NOγ, and O3 . Science. 1998;279:1339–1341. doi: 10.1126/science.279.5355.1339. - DOI - PubMed
- Ravishankara A. R., Daniel J. S., Portmann R. W.. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science. 2009;326:123–125. doi: 10.1126/science.1176985. - DOI - PubMed
-
- Davidson E. A., Kanter D.. Inventories and Scenarios of Nitrous Oxide Emissions. Environ. Res. Lett. 2014;9:105012. doi: 10.1088/1748-9326/9/10/105012. - DOI
-
-
For some selected examples, see:
- Jabłońska M., Palkovits R.. It Is No Laughing Matter: Nitrous Oxide Formation in Diesel Engines and Advances in its Abatement over Rhodium-Based Catalyst. Catal. Sci. Technol. 2016;6:7671–7687. doi: 10.1039/C6CY01126H. - DOI
- Deeba R., Molton F., Chardon-Noblat S., Costentin C.. Effective Homogeneous Catalysis of Electrochemical Reduction of Nitrous Oxide to Dinitrogen at Rhenium Carbonyl Catalyst. ACS Catal. 2021;11:6099–6103. doi: 10.1021/acscatal.1c01197. - DOI
- Ko B. H., Hasa B., Shin H., Zhao Y., Jiao F.. Electrochemical Reduction of Gaseous Nitrogen Oxides on Transition Metals at Ambient Conditions. J. Am. Chem. Soc. 2022;144:1258–1266. doi: 10.1021/jacs.1c10535. - DOI - PubMed
- Konsolakis M.. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015;5:6397–6421. doi: 10.1021/acscatal.5b01605. - DOI
- Kjellberg M., Ohleier A., Thuéry P., Nicolas E., Anthore-Dalion L., Cantat T.. Photocatalytic Deoxygenation of N-O Bonds with Rhenium Complexes: From the Reduction of Nitrous Oxide to Pyridine N-Oxides. Chem. Sci. 2021;12:10266–10272. doi: 10.1039/D1SC01974K. - DOI - PMC - PubMed
- Kapteijn F., Rodriguez-Mirasol J., Moulijn J. A.. Heterogeneous Catalytic Decomposition of Nitrous Oxide. Appl. Catal., B. 1996;9:25–64. doi: 10.1016/0926-3373(96)90072-7. - DOI
- López J. C., Quijano G., Souza T. S. O., Estrada J. M., Lebrero R., Muñoz R.. Biotechnologies for Greenhouse Gases (CH4, N2O, and CO2) Abatement: State of the Art and Challenges. Appl. Microbiol. Biotechnol. 2013;97:2277–2303. doi: 10.1007/s00253-013-4734-z. - DOI - PubMed
- Denisova K. O., Ilyin A. A., Rumyantsev R. N., Ilyin A. P., Volkova A. V.. Nitrous Oxide: Production, Application, and Protection of the Environment. Russ. J. Gen. Chem. 2019;89:1338–1346. doi: 10.1134/S107036321906032X. - DOI
- Frutos O. D., Quijano G., Aizpuru A., Muñoz R.. A State-of-the-Art Review on Nitrous Oxide Control from Waste Treatment and Industrial Sources. Biotechnol. Adv. 2018;36:1025–1037. doi: 10.1016/j.biotechadv.2018.03.004. - DOI - PubMed
- Nirisen Ø., Waller D., Brackenbury D. M.. The Development of N2O Abatement Catalyst: from Laboratory Scale to Plant Testing. Top. Catal. 2019;62:1113–1125. doi: 10.1007/s11244-018-1076-1. - DOI
- Martinez J. L., Schneider J. E., Anferov S. W., Anderson J. S.. Electrochemical Reduction of N2O with a Molecular Copper Catalyst. ACS Catal. 2023;13:12673–12680. doi: 10.1021/acscatal.3c02658. - DOI - PMC - PubMed
- Stanley J. S., Wang X. S., Yang J. Y.. Selective Electrocatalytic Reduction of Nitrous Oxide to Dinitrogen with an Iron Porphyrin Complex. ACS Catal. 2023;13:12617–12622. doi: 10.1021/acscatal.3c02707. - DOI
- Deeba R., Chardon-Noblat S., Costentin C.. Homogeneous Molecular Catalysis of the Electrochemical Reduction of N2O to N2: Redox vs. Chemical Catalysis. Chem. Sci. 2021;12:12726–12732. doi: 10.1039/D1SC03044B. - DOI - PMC - PubMed
- Piper S. E. H., Casadevall C., Reisner E., Clarke T. A., Jeuken L. J. C., Gates A. J., Butt J. N.. Photocatalytic Removal of the Greenhouse Gas Nitrous Oxide by Liposomal Microreactors. Angew. Chem. Int. Ed. 2022;61:e202210572. doi: 10.1002/anie.202210572. - DOI - PMC - PubMed
- Zhuang Z., Guan B., Chen J., Zheng C., Zhou J., Su T., Chen Y., Zhu C., Hu X., Zhao S., Guo J., Dang H., Zhang Y., Yuan Y., Yi C., Xu C., Xu B., Zeng W., Li Y., Shi K., He Y., Wei Z., Huang Z.. Review of Nitrous Oxide Direct Catalytic Decomposition and Selective Catalytic Reduction Catalysts. Chem. Eng. J. 2024;486:150374. doi: 10.1016/j.cej.2024.150374. - DOI
- Wu D., Chen K., Lv P., Ma Z., Chu K., Ma D.. Direct Eight-Electron N2O Electroreduction to NH3 Enabled by an Fe Double-Atom Catalyst. Nano Lett. 2024;24:8502–8509. doi: 10.1021/acs.nanolett.4c00576. - DOI - PubMed
- Anthore-Dalion L., Nicolas E., Cantat T.. Catalytic Metal-Free Deoxygenation of Nitrous Oxide with Disilanes. ACS Catal. 2019;9:11563–11567. doi: 10.1021/acscatal.9b04434. - DOI
-
-
- Kumar A., Gao C.. Homogeneous (De)hydrogenative Catalysis for Circular Chemistry – Using Waste as a Resource. ChemCatChem. 2021;13:1105–1134. doi: 10.1002/cctc.202001404. - DOI
- Keijer T., Bakker V., Slootweg J. C.. Circular Chemistry to Enable a Circular Economy. Nat. Chem. 2019;11:190–195. doi: 10.1038/s41557-019-0226-9. - DOI - PubMed
- Genoux A., Severin K.. Nitrous Oxide as Diazo Transfer Reagent. Chem. Sci. 2024;15:13605–13617. doi: 10.1039/D4SC04530K. - DOI - PMC - PubMed
- Severin K.. Synthetic Chemistry with Nitrous Oxide. Chem. Soc. Rev. 2015;44:6375–6386. doi: 10.1039/C5CS00339C. - DOI - PubMed
- Severin K.. Homogeneous Catalysis with Nitrous Oxide. Trends Chem. 2023;5:574–576. doi: 10.1016/j.trechm.2022.12.008. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
