Multiple sclerosis: 2024 update
- PMID: 40636815
- PMCID: PMC12238822
- DOI: 10.17879/freeneuropathology-2025-6762
Multiple sclerosis: 2024 update
Abstract
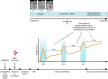
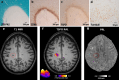
Multiple sclerosis (MS) is a complex immune-mediated disease that leads to neurological disability, with ongoing challenges in understanding its initiation, predicting progression, and optimizing personalized treatment. This review article summarizes key research findings from 2024, covering advances in diagnostic criteria, understanding of pathophysiology, and treatment strategies. New studies reinforce the strong link between Epstein-Barr virus (EBV) and MS, while recent data point towards a role of genetics in MS disease progression. The 2024 McDonald criteria revision enhances diagnostic specificity and includes novel MRI markers and facilitates measurement of cerebrospinal fluid biomarkers. Additionally, recent genetic discoveries, advanced imaging techniques, and emerging biomarkers are refining disease monitoring and prognosis. Finally, we highlight promising therapeutic developments, including Bruton Tyrosine Kinase (BTK) inhibitors and CAR T-cell therapies, with the former representing a paradigm shift in the potential of targeting MS progression beyond focal inflammation.
Keywords: Biomarkers; Diagnostic criteria; Disease course; Genetics; Imaging; Multiple sclerosis; Treatment.
© 2025 The author(s).
Conflict of interest statement
L.K. receives research support from the German Research Foundation (DFG), the Interdisciplinary Center for Clinical Research (IZKF) Münster, National MS Society, Biogen, Novartis and Merck Serono. She received compensation for serving on scientific advisory boards and speaker honoraria from Alexion, Amgen, Argenx, Bayer, Biogen, Bristol-Myers Squibb, Grifols, Hexal, Horizon, Janssen, Merck Serono, Novartis, Roche, Sandoz, Sanofi, Santhera, Teva and Viatris. L.A. receives grants from the Research Council of Finland, Aatos Erkko Foundation, US National MS Society, MerckSerono and Sanofi. She received speaker and advising honoraria from Sanofi, Biogen, Novartis, Kiniksa, Continuum Therapeutics and Merck. M.S. declares no conflicts of interest. T.K. receives research funding from the German Research Foundation, Interdisciplinary Center for Clinical Research (IZKF) Münster, National MS Society, German MS Society and Novartis. She received compensation for serving on scientific advisory boards from Novartis, Sanofi and Merck and speaker honoraria from Novartis, Biogen, Sanofi and Roche.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
