Nuclear Receptor ERRγ Protects Against Cardiac Ischemic Injury by Suppressing GBP5-Mediated Myocardial Inflammation
- PMID: 40636987
- PMCID: PMC12242898
- DOI: 10.1096/fj.202500763R
Nuclear Receptor ERRγ Protects Against Cardiac Ischemic Injury by Suppressing GBP5-Mediated Myocardial Inflammation
Abstract
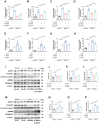
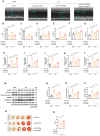
Myocardial inflammation plays a critical role in the progression of injury following myocardial infarction (MI), yet the transcriptional mechanisms regulating cardiomyocyte inflammation to mitigate post-ischemic injury remain poorly understood. This study elucidated the role of Estrogen-Related Receptor Gamma (ERRγ) in modulating the inflammatory response post-MI, demonstrating that ERRγ expression was downregulated in ischemic tissue and hypoxic neonatal mouse ventricular myocytes (NMVMs). Cardiomyocyte-specific overexpression of ERRγ reduced infarct size, improved cardiac function, and suppressed excessive myocardial inflammation and pyroptosis by binding to the GBP5 promoter, thereby inhibiting GBP5 transcription and reducing NLRP3 inflammasome assembly. The protective effects of ERRγ overexpression were reversed by overexpressing GBP5, and the ERRγ agonist DY131 also improved cardiac function after MI. These findings suggest that ERRγ activation reduces myocardial ischemic injury by regulating cardiomyocyte inflammation and pyroptosis, highlighting ERRγ as a potential novel therapeutic target for attenuating post-MI injury.
Keywords: ERRγ; GBP5; inflammation; myocardial infarction; pyroptosis.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Reed G. W., Rossi J. E., and Cannon C. P., “Acute Myocardial Infarction,” Lancet 389 (2017): 197–210. - PubMed
MeSH terms
Substances
Grants and funding
- 82130009/the State Key Program of National Natural Science Foundation of China
- 82070515/MOST | National Natural Science Foundation of China (NSFC)
- 82370317/MOST | National Natural Science Foundation of China (NSFC)
- 19411963500/Shanghai City Committee of Science and Technology Research Projects
- YG2023QNB12/MOE | Fundamental Research Funds for the Central Universities (Fundamental Research Fund for the Central Universities)
LinkOut - more resources
Full Text Sources
Medical

