Pharmacovigilance study for the identification of mogamulizumab-induced immune-related adverse events using a real-world database
- PMID: 40637043
- PMCID: PMC12242157
- DOI: 10.1093/oncolo/oyaf201
Pharmacovigilance study for the identification of mogamulizumab-induced immune-related adverse events using a real-world database
Abstract
Background: Mogamulizumab is a humanized anti-CCR4 monoclonal antibody used for relapsed/refractory adult T-cell leukemia, cutaneous T-cell lymphoma, and/or Sézary syndrome. Reports of immune-related adverse events (irAEs) in these patients are increasing, and the association between irAEs and mogamulizumab remains to be elucidated. This study aimed to evaluate the association between mogamulizumab and immune-related adverse events (irAEs), as well as to characterize the irAEs associated with mogamulizumab using data from a large-scale spontaneous reporting system.
Methods: We performed an exploratory hypothesis-generating analysis of patients from 1967 to September 2023 using VigiBase, a World Health Organization spontaneous adverse event reporting system database. We performed a disproportionality analysis and determined the reporting odds ratios and information components between the drugs of interest and each irAE.
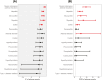
Results: Mogamulizumab was associated with some irAEs, including myocarditis, severe cutaneous adverse reactions, hepatitis, and myositis. Mogamulizumab exhibited significantly higher reporting rates of these 4 irAEs compared to the anticancer agents other than mogamulizumab. Conversely, the reporting rate of other irAEs, including endocrine autoimmune diseases induced by immune checkpoint inhibitors, was not significant in patients who received mogamulizumab.
Conclusions: Mogamulizumab is associated with irAEs, including myocarditis, severe cutaneous adverse reactions, hepatitis, and myositis.
Keywords: VigiBase; disproportionality analysis; irAEs; mogamulizumab; sézary syndrome.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
Authors declare no competing interests.
Figures

References
-
- Kim YH, Bagot M, Pinter-Brown L, et al. ; MAVORIC Investigators. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192-1204. https://doi.org/ 10.1016/S1470-2045(18)30379-6 - DOI - PubMed
-
- Fujikawa K, Saito T, Kurose K, et al. Integrated analysis of phase 1a and 1b randomized controlled trials; Treg-targeted cancer immunotherapy with the humanized anti-CCR4 antibody, KW-0761, for advanced solid tumors. PLoS One. 2023;18:e0291772. https://doi.org/ 10.1371/journal.pone.0291772 - DOI - PMC - PubMed
-
- Hong DS, Rixe O, Chiu VK, et al. Mogamulizumab in combination with nivolumab in a phase I/II study of patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2022;28:479-488. https://doi.org/ 10.1158/1078-0432.CCR-21-2781 - DOI - PMC - PubMed
-
- Maeda Y, Wada H, Sugiyama D, et al. Depletion of central memory CD8(+) T cells might impede the antitumor therapeutic effect of Mogamulizumab. Nat Commun. 2021;12:7280. https://doi.org/ 10.1038/s41467-021-27574-0 - DOI - PMC - PubMed
-
- de Masson A, Darbord D, Dobos G, et al. Macrophage-derived CXCL9 and CXCL11, T-cell skin homing, and disease control in mogamulizumab-treated CTCL patients. Blood. 2022;139:1820-1832. https://doi.org/ 10.1182/blood.2021013341 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

