Mitochondrial Dysfunction and Defects in Mitochondrial Adaptation to Exercise Training in the Muscle of Patients With COPD: Disease Versus Disuse
- PMID: 40637117
- PMCID: PMC12243074
- DOI: 10.1111/apha.70079
Mitochondrial Dysfunction and Defects in Mitochondrial Adaptation to Exercise Training in the Muscle of Patients With COPD: Disease Versus Disuse
Abstract
Aim: Chronic obstructive pulmonary disease (COPD) is frequently associated with skeletal muscle dysfunction, having a considerable impact on exercise tolerance and patient prognosis. Mitochondria play a role in skeletal muscle weakness and exercise intolerance in COPD, but the majority of studies on mitochondrial function are biased by the fact that physical activity is greater in healthy subjects than in patients. Furthermore, exercise training (ET) has been proposed as a therapeutic strategy to prevent skeletal muscle dysfunction in COPD, but very few results are available on mitochondrial adaptation in response to ET.
Methods: Skeletal muscle mitochondrial function and the potential efficacy of ET on this function were compared between 12 patients with COPD and 21 healthy subjects with similar low levels of physical activity. Various markers of mitochondrial respiration, oxidative stress, biogenesis, and dynamics were assessed.
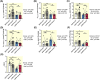
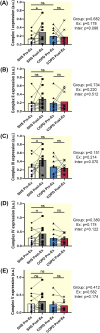
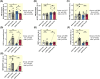
Results: Lower oxidative phosphorylation (OxPhos; p < 0.001) and increased nonphosphorylating respiration (p = 0.025) and mitochondrial oxidative damage (lipid peroxidation (p = 0.014) and protein carbonylation (p = 0.020)) were observed in patients. While ET increased OxPhos efficiency (p = 0.011) and reduced nonphosphorylating respiration (p < 0.001) and lipid peroxidation (p < 0.001) in patients' muscle mitochondria, it fails to improve maximal respiration (p = 0.835) and expression of the antioxidant enzyme MnSOD (p = 0.606), mitochondrial transcription factor TFAM (p = 0.246), and mitochondrial complexes I, III, and IV (p = 0.816, p = 0.664, p = 0.888, respectively) as observed in healthy subjects.
Conclusion: The mitochondrial dysfunction and the defects in mitochondrial adaptation to ET that we observe in the muscle of patients with COPD are intrinsic to the disease and do not arise from muscle disuse.
Keywords: COPD; exercise; mitochondrial dysfunction; muscle; oxidative stress; pulmonary rehabilitation.
© 2025 The Author(s). Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Meyer A., Zoll J., Charles A. L., et al., “Skeletal Muscle Mitochondrial Dysfunction During Chronic Obstructive Pulmonary Disease: Central Actor and Therapeutic Target,” Experimental Physiology 98, no. 6 (2013): 1063–1078. - PubMed
-
- Puente‐Maestu L., Pérez‐Parra J., Godoy R., et al., “Abnormal Mitochondrial Function in Locomotor and Respiratory Muscles of COPD Patients,” European Respiratory Journal 33, no. 5 (2009): 1045–1052. - PubMed
-
- Naimi A. I., Bourbeau J., Perrault H., et al., “Altered Mitochondrial Regulation in Quadriceps Muscles of Patients With COPD,” Clinical Physiology and Functional Imaging 31, no. 2 (2011): 124–131. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

