Biomarker testing implementation for molecularly targeted therapy in non-small cell lung cancer patients
- PMID: 40637305
- PMCID: PMC12314203
- DOI: 10.1177/03008916251341996
Biomarker testing implementation for molecularly targeted therapy in non-small cell lung cancer patients
Abstract
Background: Recent advancements in identifying druggable molecular drivers in lung adenocarcinoma (LUAD), have transformed treatment paradigms. In recent years, Next Generation Sequencing (NGS) has gained momentum as an essential tool for in-depth simultaneous analysis of multiple genes, thereby streamlining the diagnostic process in LUAD. Despite this, the implementation of NGS testing in both the US and Europe remains suboptimal.
Aims: In compliance with a decree issued by the Italian Ministry of Health, Lombardy Region recently launched an initiative to implement NGS testing in patients with advanced LUAD. In this context, a real-world prospective observational study was planned to assess the efficacy of the regional network of molecular laboratories in testing nine biomarkers (KRAS p.G12C, EGFR, BRAF, HER2, MET mutations; ALK, ROS1, NTRK1-3, RET rearrangements), for on-label molecularly targeted drugs.
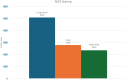
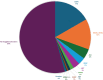
Results: In 2023, out of the 2784 advanced/metastatic LUAD patients expected in Lombardy, 2343 (84.2%) were successfully evaluated with an NGS panel including all the nine biomarkers for on-label drugs. Actionable aberrations were identified in 45.5% of the patients (1068/2343), predominantly involving EGFR, KRAS, and ALK genes.
Conclusion: Our data provide evidence that establishing a structured network of NGS hubs is mandatory to ensure access of advanced LUAD patients to molecularly targeted treatments.
Keywords: Lung adenocarcinoma; NGS; precision oncology; target therapy.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Bade BC, Dela Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med 2020; 41: 1-24. - PubMed
-
- Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med 2021; 27: 1345-1356. - PubMed
-
- Mosele MF, Westphalen CB, Stenzinger A, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol 2024; 35: 588-606. - PubMed
-
- Al-Ahmadi A, Ardeshir-Larijani F, Fu P, et al. Next generation sequencing of advanced non-small cell lung cancer: utilization based on race and impact on survival. Clin Lung Cancer 2021; 22: 16-22.e1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous