Intravenous Bronchodilators in Pediatric Critical Asthma: A Systematic Review and Network Meta-Analysis
- PMID: 40637351
- PMCID: PMC12243718
- DOI: 10.1002/ppul.71192
Intravenous Bronchodilators in Pediatric Critical Asthma: A Systematic Review and Network Meta-Analysis
Abstract
Introduction: Pediatric critical asthma is one of the most common pediatric illnesses in children admitted to the pediatric ward and pediatric intensive care unit (PICU). Adjunct intravenous (IV) bronchodilators are often used when initial management with systemic corticosteroids and inhaled short-acting beta agonists (SABA) fail to provide improvement in a patient's clinical condition. While the recent guidelines gave recommendations for the use of different IV bronchodilators compared to placebo, it did not include ranking on which one should be used as first-line or second-line agent. The aim of this network meta-analysis is to determine the effect of IV bronchodilators on patient-centered outcomes and rank medications based on their effectiveness in these outcomes.
Methods: A systematic review was conducted using three databases MEDLINE, Embase, and CINAHL to identify randomized control trials examining the use of IV magnesium sulfate (MgSO4), IV methylxanthines (aminophylline or theophylline), IV SABA (salbutamol, terbutaline) in pediatric critical asthma patients. Bayesian network metanalytic framework was used to compare the interventions. Results are reported as odds ratio (OR) or mean difference (MD) and 95% Credible Interval (CrI).
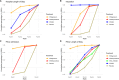
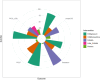
Results: Twelve trials (n = 852) were included in the network meta-analysis. Largest reduction in hospital length of stay (LOS), PICU admission, and PICU LOS were noted with IV MgSO4; (MD: -3.1 days, 95% CrI: -6.9 days to 0.13 days), (OR 0.21; 95% CrI 0.02, 1.3), and (MD: -4.0 days, 95% CrI: -7.1 days to -1.2 days) respectively. IV MgSO4 was ranked first in three outcomes of interest with Surface Under the Cumulative Ranking curve (SUCRA) of 0.884 for hospital LOS, 0.919 for PICU admission, and 0.957 for PICU LOS. For preventing intubation, IV SABA was ranked the highest (SUCRA 0.995), but the only study with IV SABA had zero intubation events. In a sensitivity analysis that excluded studies with zero events, the intubation rate was lowest with IV MgSO4 (OR 0.10; 95% CrI 0.003, 0.88) and it was ranked the best treatment (SUCRA 0.921).
Conclusions: In this network meta-analysis comparing different IV adjunct bronchodilators, IV MgSO4 was ranked first followed by IV SABA, and then IV methylxanthines. Given these findings and the favorable safety profile, ease of use, and low cost, IV MgSO4 appears most promising the first adjunct IV bronchodilator, however, further large high-quality trials are still needed before it can be endorsed as routine first-line agent.
Keywords: aminophylline; bronchodilator; children; critical asthma; magnesium sulfate; methylxanthines; network meta‐analysis; salbutamol; status asthmaticus; terbutaline.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Rehder K. J., “Adjunct Therapies for Refractory Status Asthmaticus in Children,” Respiratory Care 62, no. 6 (2017): 849–865. - PubMed
-
- Rogerson C. M., White B. R., and Abu‐Sultaneh S., “Pharmacological Management of Pediatric Critical Asthma,” Respiratory Care 70, no. 6 (2025): 746–759, https://pubmed.ncbi.nlm.nih.gov/39348943/. - PubMed
-
- Rogerson C. M., Hogan A. H., Waldo B., White B. R., Carroll C. L., and Shein S. L., “Wide Institutional Variability in the Treatment of Pediatric Critical Asthma: A Multicenter Retrospective Study,” Pediatric Critical Care Medicine: A Journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 25, no. 1 (2024): 37–46. - PubMed
-
- White B. R., Miller A. G., Baker J., et al., “AARC and PALISI Clinical Practice Guideline: Pediatric Critical Asthma,” Respiratory Care 70, no. 5 (2025): 593–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

