The magnitude and time course of pre-saccadic foveal prediction depend on the conspicuity of the saccade target
- PMID: 40637717
- PMCID: PMC12245172
- DOI: 10.7554/eLife.91236
The magnitude and time course of pre-saccadic foveal prediction depend on the conspicuity of the saccade target
Abstract
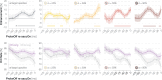
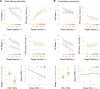
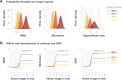
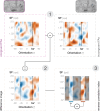
We previously demonstrated that during the preparation of a saccadic eye movement, human observers anticipate defining features of the eye movement target in pre-saccadic foveal vision (Kroell and Rolfs, 2022). In this Research Advance, we show that the conspicuity of feature information at the saccade target location influences the magnitude and time course of foveal enhancement. Observers prepared a saccade to a peripheral orientation signal (the target) while monitoring the appearance of another orientation signal (the probe) in their pre-saccadic center of gaze. The foveal probe appeared in 50% of trials and either had the same orientation as the target (congruent) or a different orientation (incongruent). Crucially, we manipulated the opacity of the target against the 1/f noise background in four logarithmic steps (25-90%). An increase in opacity translated to an increase in luminance contrast and signal-to-noise ratio of orientation information within the target region. Foveal enhancement defined as the difference between hit rates to target-congruent and target-incongruent foveal probes increased with target opacity. Moreover, the time course of foveal enhancement showed an oscillatory pattern that was particularly pronounced at high target opacities. Reverse correlations furthermore suggest that at higher target opacities, false alarms were increasingly triggered by signal, i.e., by incidental orientation information in the foveal noise. Beyond providing new mechanistic insights, these findings are relevant for researchers planning to adapt our paradigm to study related questions. Presenting the saccade target at a high signal-to-noise ratio appears beneficial, as foveal congruency effects, especially when time-resolved, are most robustly detectable.
Keywords: active vision; foveal vision; human; neuroscience; psychophysics; saccadic eye movements.
© 2023, Kroell and Rolfs.
Conflict of interest statement
LK, MR No competing interests declared
Figures




Update of
- doi: 10.1101/2023.07.06.548056
- doi: 10.7554/eLife.91236.1
- doi: 10.7554/eLife.91236.2
- doi: 10.7554/eLife.91236.3
Similar articles
-
Pre-saccadic shifts of attention in individuals diagnosed with schizophrenia.Brain Behav. 2024 Mar;14(3):e3466. doi: 10.1002/brb3.3466. Brain Behav. 2024. PMID: 38450916 Free PMC article.
-
Attention modulates subjective time perception across eye movements.Vision Res. 2025 Feb;227:108540. doi: 10.1016/j.visres.2025.108540. Epub 2025 Jan 7. Vision Res. 2025. PMID: 39778360
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

