Evaluating finger-prick blood collection for remote quantification of neurofilament light in neurological diseases
- PMID: 40637865
- PMCID: PMC12245952
- DOI: 10.1007/s00415-025-13232-8
Evaluating finger-prick blood collection for remote quantification of neurofilament light in neurological diseases
Abstract
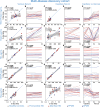
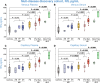
Promising blood-based biomarkers of neuropathology have emerged with potential for therapeutic development and disease monitoring. However, these tools will require specialist tertiary services for integration into clinical management. Remote sampling for biomarker assessment could reduce burden of in-person clinical visits for such tests as well as increasing the sampling frequency and patient geographical outreach. Here, we evaluated a finger-prick blood collection approach for remote quantification of neurofilament light (NfL), a candidate blood-based biomarker evident in various neurological disorders, and other exploratory markers of neuronal injury and neuroinflammation (GFAP, tau). Matched samples from venepuncture and finger-prick were collected and processed into plasma and/or serum to directly compare analyte levels from a multi-disease discovery cohort (n = 54 healthy controls; n = 57 Huntington's disease (HD); n = 34 multiple sclerosis; n = 7 amyotrophic lateral sclerosis; n = 11 Parkinson's disease), and a HD confirmatory cohort (n = 57 healthy controls; n = 64 HD). Two delayed processing conditions were compared, three- and seven-day delay, simulating ambient shipment. Capillary NfL and GFAP concentrations were equivalent to those in venous serum and plasma in the multi-disease discovery cohort and HD confirmatory cohort. Only NfL remained stable after a seven-day processing delay in both venous and capillary serum samples. Using NfL concentrations from capillary blood, we replicated previously published disease group differences measured in venous blood. This data supports our finger-prick approach for remote collection and quantification of NfL. With the widespread applications for NfL across the spectrum of neurological disorders, this has the potential to transform disease monitoring, prognosis, and therapeutic development within clinical practice and research.
Keywords: Biomarkers; Blood; Finger-prick; Neurodegenerative disorders; Neurofilament light; Remote sampling.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: LMB holds consultancy contracts with Annexon Biosciences, Remix Therapeutics, PTC Therapeutics, Alchemab Therapeutics, Latus Bio, and LoQus23 Therapeutics Ltd via UCL Consultants Ltd. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). AN received consultancy fees during the design phase of AccessPD and reports consultancy and personal fees from AstraZeneca, AbbVie, Profile, Roche, Biogen, UCB, Bial, Charco Neurotech, Alchemab, Sosei Heptares and Britannia, outside the submitted work. AN is an Associate Editor for the Journal of Parkinson’s Disease. In the last 3 years, JC has received support from the Health Technology Assessment (HTA) Programme (National Institute for Health Research, NIHR), the UK MS Society, the US National MS Society and the Rosetrees Trust. He is supported in part by the NIHR University College London Hospitals (UCLH) Biomedical Research Centre, London, UK. He has been a local principal investigator for a trial in MS funded by MS Canada. A local principal investigator for commercial trials funded by: Ionis and Roche; and has taken part in advisory boards/consultancy for Biogen, Contineum Therapeutics, Lucid, Merck, NervGen, Novartis and Roche. SJT reports that over the past 3 years consultancy fees for advisory services were paid to University College London Consultants, a wholly-owned subsidiary of University College London from the following companies: Alchemab, Alnylam Pharmaceuticals, Annexon Bioscience, Arrowhead, Atalanta Therapeutics, Biogen, Catapult, Design Therapeutics, F. Hoffman-La Roche, Ipsen, Iris Medicine, Latus Bio, LifeEdit, Novartis Pharma, Pfizer, Prilenia, Prime Global, PTC Therapeutics, Remix, Rgenta Therapeutics, SkyHawk, Takeda Pharmaceuticals, Triplet Therapeutics, UniQure Biopharma, Vertex Pharmaceuticals, Vico Therapeutics, Wave Life Sciences. SJT has also consulted for Abingworth, Ascidian Therapeutics, EQT, Cure Ventures, FunctionRX, Harrisson & Star, and IQVIA through the office of Celtic Phenomenon. In the past 3 years, University College London Hospitals NHS Foundation Trust, Professor Tabrizi’s host clinical institution, received funding to run clinical trials for Alnylam Pharmaceuticals, F. Hoffman-La Roche, Novartis Pharma, PTC Therapeutics, and UniQure Biopharma. Ethical approval: This study complies with the Declaration of Helsinki and was approved by local ethics committees including The National Hospital for Neurology and Neurosurgery and the Institute of Neurology Joint Research Ethics Committee (HD and controls; ref: 03/N008); London—City & East Research Ethics committee (ALS, MS; ref: 09/H0703/27); South West – Central Bristol Research Ethics Committee (PD: PR 18/SW/0255). Consent to participate: All participants within the study gave written informed consent before blood collection.
Figures




References
-
- Dehnel T (2013) The European dementia prevention initiative. Lancet Neurol 12(3):227–228 - PubMed
-
- Wilson E, Leyrat C, Baraitser P, Free C (2019) Does internet-accessed STI (e-STI) testing increase testing uptake for chlamydia and other STIs among a young population who have never tested? Secondary analyses of data from a randomised controlled trial. Sex Transm Infect 95(8):569–574 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

