The PD COMM Process Evaluation: Describing Interventions and Implementation in a UK Pragmatic Randomised Controlled Trial of Speech and Language Therapy for People With Parkinson's-Related Dysarthria
- PMID: 40637984
- PMCID: PMC12244302
- DOI: 10.1111/1460-6984.70084
The PD COMM Process Evaluation: Describing Interventions and Implementation in a UK Pragmatic Randomised Controlled Trial of Speech and Language Therapy for People With Parkinson's-Related Dysarthria
Abstract
Background: As people with Parkinson's experience progressive communication changes, effective, implementable speech and language therapy (SLT) interventions are needed. Process evaluations alongside pragmatic randomised controlled trials (RCTs) are of clinical value if they describe, compare and understand the implementation of trial interventions. This paper reports the PD COMM process evaluation. PD COMM was a large, UK multi-centre phase III pragmatic RCT of SLT in the National Health Service (NHS). It recruited 388 people with Parkinson's who were randomised to Lee Silverman Voice Treatment (LSVT), Standard NHS SLT, or no dysarthria intervention.
Aims: To describe and compare the content and service delivery components of the PD COMM SLT interventions; understand experiences of implementing LSVT; explain trial outcomes; and reflect on implications for practice and research.
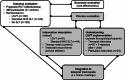
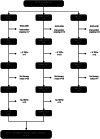
Methods and procedures: We took a pragmatic, mixed methods approach. The intervention description team used a sub-sample of routine therapy notes and trial record forms, the Template for Intervention Description and Replication (TIDieR) and simple descriptive statistics to compare Individual Participant Therapy Data (LSVT n = 51; Standard NHS SLT n = 54). In parallel, informed by Normalisation Process Theory (NPT), the implementation team conducted qualitative interviews with a sub-sample of therapists (n = 20) and participants (n = 24) to understand the additional work of implementing LSVT. The core process evaluation team met to integrate the findings in relation to the trial outcomes.
Outcomes and results: LSVT was largely delivered per protocol, tailored to participants' interests and interactions. Dosage was a key difference between the two interventions, commonly achieved by two or more therapists delivering LSVT. Effective mechanisms were LSVT's structured design, repetitive and social nature, practise requirements and focus on volume. Standard NHS SLT was eclectic, reflecting a range of clinical approaches at a lower intensity, including some techniques and activities in common with LSVT. Although focused on impairment therapy, including specific voice therapy techniques, it also featured cognitive-linguistic and psychosocial targets and low technology augmentative and alternative communication (AAC). The trial design may have limited opportunities for group intervention.
Conclusions and implications: Any LSVT roll-out needs service support and coordination, and should take an inclusive approach. Future research of Standard NHS SLT should explore a rationale for dosage and more explicit tailoring to individuals and their families. There is also a pressing need to deliver the benefits of LSVT in a cost-effective manner and to develop a range of evidence-based, implementable alternatives as people's communication support needs change.
What this paper adds: What is already known on the subject Lee Silverman Voice Treatment (LSVT) has a body of incrementally-developed evidence from effectiveness trials but has not previously been tested in a pragmatic randomised controlled trial (RCT) with an embedded process evaluation. What this paper adds to the existing knowledge This mixed methods process evaluation paper describes and compares content and service delivery components to understand similarities and differences between LSVT and Standard NHS SLT interventions and experiences of implementing LSVT in the UK NHS. What are the potential or actual clinical implications of this work? Services can use the findings to plan delivery of intensive interventions and to reflect on the content and service delivery aspects of locally Standard NHS SLT and how it might be improved.
Keywords: Parkinson disease; implementation science; intervention study; pragmatic clinical trial; randomized controlled trial; speech and language therapy.
© 2025 The Author(s). International Journal of Language & Communication Disorders published by John Wiley & Sons Ltd on behalf of Royal College of Speech and Language Therapists.
Conflict of interest statement
Avril Nicoll and Gillian Beaton received training in LSVT LOUD from LSVT Global. Marian Brady, Christina H Smith and Gillian Beaton are speech and language therapists, and Avril Nicoll was a speech and language therapist until 31 July 2023.
Figures


References
-
- Baylor, C. , Cook K. J., and Mcauliffe M. J.. 2024. “Take Us Into Account: Perspectives of Family Members of People With Parkinson's Disease Regarding Speech‐Language Pathology Intervention.” American Journal of Speech‐Language Pathology 33: 736–755. - PubMed
-
- Boa, S. , Duncan E., Haraldsdottir E., and Wyke S.. 2018. “Patient‐Centred Goal Setting in a Hospice: A Comparative Case Study of How Health Practitioners Understand and Use Goal Setting in Practice.” International Journal of Palliative Nursing 24: 115–122. - PubMed
-
- Brown, E. V. D. , Wallace S. E., and Liu Q.. 2021. “Speech‐Language Pathologists' Practice Patterns When Designing Home Practice Programs for Persons With Aphasia: A Survey.” American Journal of Speech‐Language Pathology 30: 2605–2615. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

