Real-World Experience with Lifitegrast Ophthalmic Solution in Patients with Dry Eye Disease: A Provider Survey in the USA and Canada
- PMID: 40638059
- PMCID: PMC12370593
- DOI: 10.1007/s40123-025-01190-3
Real-World Experience with Lifitegrast Ophthalmic Solution in Patients with Dry Eye Disease: A Provider Survey in the USA and Canada
Abstract
Introduction: Lifitegrast ophthalmic solution 5% is indicated to treat signs and symptoms of dry eye disease (DED). This study assessed eyecare professionals' (ECPs) real-world experiences with lifitegrast in DED.
Methods: A total of 12 ECPs (6 ophthalmologists and 6 optometrists) with experience prescribing lifitegrast completed a cross-sectional survey on practice characteristics, lifitegrast utilization, satisfaction, and adverse events (AEs). ECPs rated satisfaction with lifitegrast overall and for specific clinical outcomes versus other prescription eye drops on a 1 (very dissatisfied) to 10 (very satisfied) Likert scale.
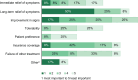
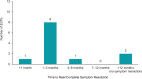
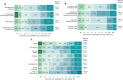
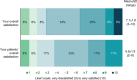
Results: ECPs reported a mean of 1288 (range, 35-6000) patients with DED treated annually, with 20.9% receiving lifitegrast. Overall, 66.7% of ECPs reported near/complete symptom resolution in patients after 1-3 months of lifitegrast treatment. Mean (range) satisfaction ratings for onset/effectiveness were 6.8 (3-9)/6.6 (3-9). Satisfaction with reduction of DED signs was generally high: increased tear film breakup time, 5.8 (3-9); reduced conjunctival/corneal staining, 6.9 (3-9); increased Schirmer test score, 6.0 (3-9); increased tear meniscus height, 6.0 (3-9); and reduced Ocular Surface Disease Index severity, 7.0 (3-10). Symptom reduction satisfaction ratings were: itching, 5.3 (1-9); dryness, 6.9 (3-9); burning/stinging, 6.3 (1-9); redness, 6.2 (4-9); pain, 6.3 (3-9); light sensitivity, 6.5 (3-9); and blurred/poor vision, 6.8 (4-9). Overall satisfaction (ECPs/patients) was rated 7.1 (3-10)/6.8 (2-9). Predominant uses of lifitegrast included contact lens-induced DED (91.7%) and DED before/after refractive or cataract surgery (83.3% each). AEs reported were consistent with the known AE profile of lifitegrast and included burning/stinging, blurred vision, and dysgeusia.
Conclusions: This real-world survey showed that ECPs use lifitegrast to treat one fifth of their patients with DED and reported moderate-to-high personal and patient satisfaction with lifitegrast treatment.
Keywords: Cross-sectional survey; Drug utilization; Dry eye syndromes; Lymphocyte function-associated antigen-1; Physician satisfaction; Signs and symptoms.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of Interest: Cecelia Koetting reports serving as a speaker, consultant, research, and/or advisory for Allergan/AbbVie, Alcon, Azura, Bausch + Lomb, Blinkjoy, Bruder, Dompe, Harrow, Myze, Orasis, PRN, Tarsus, Topcon Healthcare, Trukera Medical, and Viatris. Justin Schweitzer reports serving as a consultant or speaker for Alcon, Allergan/AbbVie, Bausch + Lomb, Bruder, Dompé, Glaukos, Iveric Bio, LKC Technologies, MediPrint Ophthalmics, Ocuphire Pharma, Reichert Technologies, ScienceBased Health, Sight Sciences, Sun Pharmaceutical Industries, Tarsus Pharmaceuticals, Théa, Topcon Healthcare, Trukera Medical, Visus Therapeutics, and Zeiss; and serving as Chief Medical Editor for Modern Optometry. Kelly K. Nichols reports receiving research funding from Aramis Biosciences, Kowa, ScienceBased Health, Sylentis, and TearScience; and serving as a consultant to and receiving honoraria from Allergan/AbbVie, Alcon, Aldeyra Therapeutics, Azura Ophthalmics, Bausch + Lomb, Bruder, Cavalry Biosciences, Dompé, HanAll Biopharma, Harrow, Novaliq, Novartis, Oyster Point Pharma (now Viatris), Sight Sciences, Sydnexis, Tarsus, TearSolutions, Théa, Topcon Healthcare, and Trukera Medical. Marguerite McDonald reports serving as a consultant to AbbVie, Alcon, Bausch + Lomb, Brill, Johnson & Johnson, Nordic Pharma, Ocuphire Pharma, Sun Pharma, Tarsus, and Trukera. Christopher E. Starr reports serving as a consultant to Alcon, Aldeyra Therapeutics, Allergan/AbbVie, Allgenesis, Amgen, Azura, Bausch + Lomb, BlephEx, Bruder Healthcare, CSI Dry Eye, Dompé, Èssiri, Eye Care International (ECI), Glaukos, Johnson & Johnson Vision, Kala Pharmaceuticals, Lumenis Be, Novaliq, Novartis, Nuvissa, Oculis, Oyster Point Pharma (now Viatris), Quidel, Sight Sciences, Sofia Biologics, Sun Pharmaceutical Industries, Tarsus, Thea Pharma, Trukera Medical, and Verséa Health; and having stock options in CSI Dry Eye, Sofia Biologics, and Nuvissa. Clara C. Chan reports serving on advisory boards, speakers bureaus, or as a consultant for AbbVie, Aequus Pharmaceuticals, Aurion Biotech, Bausch + Lomb, Johnson & Johnson, Kala Ophthalmics, Labtician Ophthalmics, Novartis, Santen Pharmaceutical, Sun Pharmaceutical Industries, Théa Pharma, and Valeo Pharma; and also has received research funding from Claris Bio and CorNeat. Mile Brujic reports serving as a speaker, consultant, researcher, and/or advisor, and received meeting support from ABB Optical Group, Alcon, Allergan/AbbVie, Art Optical, Bausch + Lomb Health, BlephEx, Contamac, CooperVision, CSEye, Dompé, Euclid, Eyevance Pharmaceuticals, Horizon Therapeutics, Johnson & Johnson Vision Care, Kala Pharmaceuticals, Luneau, Novartis, Optovue, Oyster Point Pharma, RVL, Sight Sciences, Sun Pharmaceutical Industries, Tangible Science, Tarsus, Viatris, Visionix, Walman Optical, and ZeaVision. Louis Racine reports serving on advisory boards, speakers bureaus, or as a consultant for Allergan, AbbVie, Alcon, Bausch + Lomb, Sun Pharmaceutical Industries, Théa Pharma, Staar Surgical, and Rayner Group. Francis S. Mah reports receiving grants from Allergan; reports personal fees from Shire/Takeda/Novartis; and being a consultant for Allergan, Eyevance Pharmaceuticals, Kala Pharmaceuticals, Novartis, and Sun Pharmaceutical Industries. Melissa Barnett reports serving on advisory boards or as a consultant, global ambassador, or speaker for ABB, AbbVie, Alcon, Azura Ophthalmics, Bausch + Lomb, BCLA, Bruder Healthcare, Bruno Vision, Cloudbreak Pharma, CooperVision, Dompé, Epion, Gas Permeable Lens Institute, Ocusoft, Orasis Pharmaceuticals, Percept, ScienceBased Health, Sjögren’s Foundation, STAPLE Program, Tarsus Pharmaceuticals, Théa Pharma, and Visus Therapeutics. Marjan Farid reports serving as a consultant for Bausch + Lomb and Johnson & Johnson Vision. Eric D. Donnenfeld reports serving as a consultant to Bausch + Lomb, Ocular Therapeutix, and Rayner; and reports receiving grants from Omeros. Carolina Mercado reports serving as a consultant to Bausch + Lomb. Michelle Ratay, Megan Cavet, Robert Ryan, and Joel Fain are employees of Bausch + Lomb. Lisa K. Feulner reports serving on advisory boards or as a consultant, global ambassador, or speaker for AbbVie, Alcon, Bausch + Lomb, Bruder Healthcare Company, Carl Zeiss Meditec, Inc., Glaukos, Regener-Eyes, Science Based Health, Sun Ophthalmics, Thea Pharma, and Tarsus Pharmaceuticals. Ethical Approval: This study was determined IRB-exempt by WCG IRB (Princeton, NJ, USA; IRB tracking ID 202425583). The study was exempt from IRB approval under 45 CFR § 46.104(d)(2) because the research only includes interactions involving survey procedures; and there are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data.
Figures


References
-
- Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–83. - PubMed
-
- Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. - PubMed
-
- Gomes JAP, Azar DT, Baudouin C, et al. TFOS DEWS II iatrogenic report. Ocul Surf. 2017;15(3):511–38. - PubMed
-
- Wolffsohn JS, Lingham G, Downie LE, et al. TFOS Lifestyle: impact of the digital environment on the ocular surface. Ocul Surf. 2023;28:213–52. - PubMed
LinkOut - more resources
Full Text Sources

