The HCMV tegument protein UL88 degrades MyD88 and reduces innate immune activation
- PMID: 40638072
- PMCID: PMC12363176
- DOI: 10.1128/jvi.00414-25
The HCMV tegument protein UL88 degrades MyD88 and reduces innate immune activation
Abstract
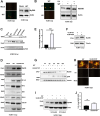
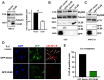
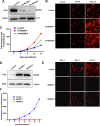
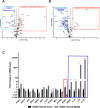
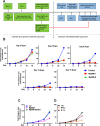
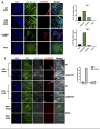
The human cytomegalovirus (HCMV) encodes the tegument protein UL88, which supports virus spread by mediating the degradation of the innate immune signaling adapter protein, myeloid differentiation primary response 88 (MyD88). MyD88 transduces signals in multiple innate immune pathways, including acting downstream of pattern recognition receptors and IL-1 cytokine family members. MyD88 is rapidly and robustly upregulated following exposure to HCMV, irrespective of viral gene expression and, even after infection, primarily within uninfected cells in a culture. However, UL88 was required to downregulate cellular MyD88 protein levels as HCMV spread through a culture. The N-terminal 181 amino acids of UL88 were required to associate with and downregulate MyD88 protein. MyD88 expression significantly suppressed virus spread by triggering the production of a heat-labile soluble factor. This factor was produced between ~3 and 6 days after initial infection and did not increase the expression of well-characterized interferon-stimulated genes (ISGs). Indeed, increased MyD88 expression downregulated the expression of almost all ISGs examined. UL88 overexpression suppressed IL-1β-induced NF-κB activation within a cell. UL88 also suppressed virus-induced translocation of NF-κB to the nucleus of uninfected neighboring cells in an infected monolayer. Furthermore, UL88 overexpression was required for effective HCMV spread following transfer of the virus from monocytes to a fibroblast monolayer. These data indicate that UL88 is a novel antagonist of the immune response that acts to enhance the natural spread of HCMV by targeting MyD88 and provides vital insight into the innate immune responses that can control HCMV spread.IMPORTANCEThe significant role of many viral genes encoded by HCMV that are not essential for replication in cell culture is often overlooked. Our study reveals the importance of UL88 for regulating the innate immune response by showing evidence for interaction with and downregulation of MyD88 protein. The UL88-dependent regulation of MyD88 is physiologically relevant, as infection is enhanced in the absence of MyD88, and spread from myeloid cells to fibroblasts is blunted in the absence of UL88. These results highlight yet another important interaction between HCMV and the immune system.
Keywords: HCMV; MyD88; UL88; immune activation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

