A noncanonical cGAS-STING pathway drives cellular and organismal aging
- PMID: 40638086
- PMCID: PMC12280946
- DOI: 10.1073/pnas.2424666122
A noncanonical cGAS-STING pathway drives cellular and organismal aging
Abstract
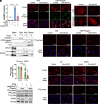
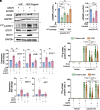
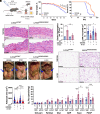
Accumulation of cytosolic DNA has emerged as a hallmark of aging, inducing sterile inflammation. Stimulator of interferon genes (STING) protein translates the sensing of cytosolic DNA by cyclic-GMP-AMP synthase (cGAS) into an inflammatory response. However, the molecular mechanisms whereby cytosolic DNA-induced cGAS-STING pathway leads to aging remain poorly understood. We show that STING does not follow the canonical pathway of activation in human fibroblasts passaged (aging) in culture, senescent fibroblasts, or progeria fibroblasts (from Hutchinson-Gilford progeria syndrome patients). Despite cytosolic DNA buildup, features of the canonical cGAS-STING pathway like increased cGAMP production, STING phosphorylation, and STING trafficking to perinuclear compartment are not observed in progeria/senescent/aging fibroblasts. Instead, STING localizes at endoplasmic reticulum, nuclear envelope, and chromatin. Despite the nonconventional STING behavior, aging/senescent/progeria cells activate inflammatory programs such as the senescence-associated secretory phenotype and the interferon response, in a cGAS and STING-dependent manner, revealing a noncanonical pathway in aging. Importantly, progeria/aging/senescent cells are hindered in their ability to activate the canonical cGAS-STING pathway with synthetic DNA, compared to young cells. This deficiency is rescued by activating vitamin D receptor signaling, unveiling mechanisms regulating the cGAS-STING pathway in aging. Significantly, in HGPS, inhibition of the noncanonical cGAS-STING pathway ameliorates cellular hallmarks of aging, reduces tissue degeneration, and extends the lifespan of progeria mice. Our study reveals that a new feature of aging is the progressively reduced ability to activate the canonical cGAS-STING pathway in response to cytosolic DNA, triggering instead a noncanonical pathway that drives senescence/aging phenotypes.
Keywords: aging; cGAS; cytosolic DNA; senescence-associated secretory phenotype; stimulator of interferon genes.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


Update of
-
A non-canonical cGAS-STING pathway drives cellular and organismal aging.bioRxiv [Preprint]. 2025 Apr 4:2025.04.03.645994. doi: 10.1101/2025.04.03.645994. bioRxiv. 2025. Update in: Proc Natl Acad Sci U S A. 2025 Jul 15;122(28):e2424666122. doi: 10.1073/pnas.2424666122. PMID: 40236012 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

