Cerebrospinal Fluid Amyloid and Tau Biomarker Changes Across the Alzheimer Disease Clinical Spectrum
- PMID: 40638114
- PMCID: PMC12246881
- DOI: 10.1001/jamanetworkopen.2025.19919
Cerebrospinal Fluid Amyloid and Tau Biomarker Changes Across the Alzheimer Disease Clinical Spectrum
Abstract
Importance: The trajectories of core Alzheimer disease (AD) cerebrospinal fluid (CSF) biomarkers and the concurrent cognitive changes across the clinical spectrum remain unclear yet are important for clinical trial design.
Objective: To map longitudinal CSF amyloid, tau, and cognitive trajectories along the clinical spectrum of AD and amyloid-negative controls.
Design, setting, and participants: This longitudinal cohort study included participants with a minimum of 2 CSF samples from Alzheimer Centrum Amsterdam cohorts across the AD clinical spectrum (ie, abnormal amyloid levels at first visit, in different clinical stages) and cognitively normal controls with initially normal CSF markers from November 2003 to July 2019. The maximum follow-up period was 19.5 years (median [IQR], 2 [0-3] years). Data were analyzed from March 2024 to May 2025.
Exposures: AD biomarkers (ß-amyloid [Aß]1-42 to Aß1-40 ratio, total tau [t-tau], and phosphorylated tau [p-tau]) detected in serially collected CSF.
Main outcomes and measures: CSF AD biomarkers were measured with Lumipulse G600II. Cognition was measured using the Mini-Mental State Examination (MMSE) and delayed memory recall component of the of the Rey Auditory Verbal Learning Test. Analysis was conducted using linear mixed models, including random intercepts and slopes, adjusting for age, education level, and sex. Each model included an interaction term of time and clinical stage to study stage-specific slopes. Biomarker conversion rates per clinical stage were studied by comparing biomarker status between visits.
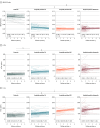
Results: The sample included 197 individuals (103 male [52.3%]), including 83 controls (mean [SD] age, 63 [8] years), 31 individuals with amyloid positivity who were cognitively unimpaired (mean [SD] age, 67 [9] years), 30 individuals with amyloid-positive mild cognitive impairment (MCI; mean [SD] age, 67 [7] years), and 53 individuals with amyloid-positive dementia (mean [SD] age, 65 [8] years). Aβ1-42/1-40 ratios decreased in controls (β [SE] = -8.55 × 10-4 [1.87 × 10-4]; P < .001) and the amyloid-positive cognitively unimpaired group (β [SE] = -1.05 × 10-3 [3.14 × 10-4]; P < .001), and remained low in amyloid-positive MCI and dementia groups. There were 10 controls (12.0%) who reached abnormal amyloid over a mean (SD) of 4.8 (3.4) years. In controls, CSF t-tau (β [SE] = 8.49 [2.55] pg/mL per year; P = .002) and p-tau (β [SE] = 1.36 [0.41] pg/mL per year; P = .001) levels increased over time, and levels also increased for those in the amyloid-positive cognitively unimpaired (t-tau: β [SE] = 17.24 [4.58] pg/mL per year; P < .001; p-tau: β [SE] = 3.10 [0.72] pg/mL per year; P < .001) and amyloid-positive MCI (t-tau: β [SE] = 30.80 [5.99] pg/mL per year; P < .001; p-tau: β [SE] = 4.40 [0.93] pg/mL per year; P < .001) groups, with t-tau increasing further in the dementia group (β [SE] = 24.97 [7.80] pg/mL per year; P = .002). Longitudinal increases in p-tau and t-tau were steeper in the amyloid-positive cognitively unimpaired and MCI groups than in controls, with 10 controls (12.0%) reaching abnormal p-tau and 12 controls (14.5%) reaching abnormal t-tau levels. Delayed recall declined most in the amyloid-positive cognitively unimpaired group (β [SE] = -0.31 [0.07]; P < .001) and was associated with CSF amyloid levels (β [SE] = 102.29 [47.30]; P = .03). MMSE scores declined most in individuals with amyloid-positive MCI (β [SE] = -1.25 [0.12]; P < .001) and dementia (β [SE] = -1.89 [0.13]; P < .001).
Conclusions and relevance: In this cohort study, CSF amyloid decreased toward abnormal levels in controls, declined further in the amyloid-positive cognitively unimpaired group , and was concurrent with decline of delayed recall; CSF amyloid stabilized in those with amyloid-positive MCI and dementia, while tau markers became increased (ie, more abnormal) in the amyloid-positive cognitively unimpaired and amyloid-positive MCI groups, suggesting that increase in CSF tau requires abnormal amyloid.
Conflict of interest statement
Figures

Similar articles
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Distinct CSF α-synuclein aggregation profiles associated with Alzheimer's disease phenotypes and MCI-to-AD conversion.J Prev Alzheimers Dis. 2025 Feb;12(2):100040. doi: 10.1016/j.tjpad.2024.100040. Epub 2025 Jan 3. J Prev Alzheimers Dis. 2025. PMID: 39863324 Free PMC article.
-
Association of Plasma Amyloid, P-Tau, GFAP, and NfL With CSF, Clinical, and Cognitive Features in Patients With Dementia With Lewy Bodies.Neurology. 2024 Jun 25;102(12):e209418. doi: 10.1212/WNL.0000000000209418. Epub 2024 Jun 3. Neurology. 2024. PMID: 38830138 Free PMC article.
-
Frequency and Clinical Outcomes Associated With Tau Positron Emission Tomography Positivity.JAMA. 2025 Jul 15;334(3):229-242. doi: 10.1001/jama.2025.7817. JAMA. 2025. PMID: 40522652
-
Cerebrospinal fluid Aβ42, t-tau, and p-tau levels in the differential diagnosis of idiopathic normal-pressure hydrocephalus: a systematic review and meta-analysis.Fluids Barriers CNS. 2017 May 10;14(1):13. doi: 10.1186/s12987-017-0062-5. Fluids Barriers CNS. 2017. PMID: 28486988 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

