Immuno-oncologyDupilumab for bullous pemphigoid related to immune checkpoint inhibitors: a retrospective case series
- PMID: 40638215
- PMCID: PMC12404300
- DOI: 10.1093/oncolo/oyaf208
Immuno-oncologyDupilumab for bullous pemphigoid related to immune checkpoint inhibitors: a retrospective case series
Abstract
Background: Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy but are associated with treatment-limiting immune-related cutaneous adverse events (irCAEs). Immune checkpoint inhibitor-related bullous pemphigoid (irBP), a severe, blistering irCAE occurs in 0.3%-1.5% of patients receiving ICI therapy. While systemic steroids can be effective, they are associated with significant toxicity and may mitigate -immunotherapy antitumor efficacy. Consequently, steroid-sparing therapies are needed. Dupilumab, an IL-4 and IL-13 receptor antagonist, has demonstrated efficacy in non-ICI-related BP and appears promising for managing irBP.
Methods: We conducted a retrospective review of patients treated with dupilumab for irBP from April 2020 to April 2024. Clinical data, outcomes, and adverse events were assessed. Inhibitor-related bullous pemphigoid response was categorized as complete response (CR), partial response (PR), or no response (NR).
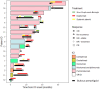
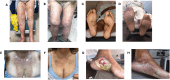
Results: In all, 17 patients (59% male, 82% non-Hispanic White; mean age 72.7 years) developed irBP while receiving PD-1/PDL-1 inhibitors. Sixteen patients (94%) received dupilumab for active irBP and one (6%) for prevention of recurrence. Dupilumab achieved CR of irBP for 12 patients (75%) and PR for 2 (12%) patients with active irBP. Ten (62%) achieved CR with dupilumab systemic monotherapy. Median time to first response was 19.5 days (range = 3-50). Most patients with CR (58%) failed prior oral corticosteroid therapy. The patient treated prophylactically experienced no irBP recurrence. Dupilumab was well-tolerated, with no adverse events.
Conclusions: Dupilumab is a promising steroid-sparing option for irBP, achieving initial response in under 20 days for most cases. Dupilumab is a valuable tool to manage this challenging irCAE while minimizing risk related to systemic steroid treatment.
Keywords: ICI; bullous pemphigoid; dupilumab; immunotherapy; oncodermatology; toxicity.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
I.N., A.M., S.D., A.M., G.I., A.I., R.M., D.I., R.O., R.K., J.D., and A.G. have no relevant disclosures. A.M. receives research funding from Amryt Pharma, Incyte Corporation, Janssen, Kintara Therapeutics, Novartis, Novocure, NIH U01, NIH U54, NIH/NCI Cancer Center Support Grant P30-CA008748; consults for ADC Therapeutics, Alira Health, Protagonist Therapeutics, OnQuality, and Janssen; and receives royalties from UpToDate. Immediate family member serves as a consultant in Ophthalmology for Adverum, Genentech, Neurogene, Novartis, Optos Plc, Outlook Therapeutics, and Regeneron outside the submitted work.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

