Behavioral state and stimulus strength regulate the role of somatostatin interneurons in stabilizing network activity
- PMID: 40638386
- PMCID: PMC12370290
- DOI: 10.1016/j.celrep.2025.115954
Behavioral state and stimulus strength regulate the role of somatostatin interneurons in stabilizing network activity
Abstract
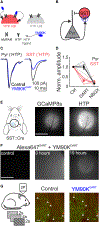
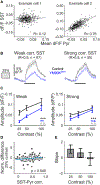
Inhibition stabilization enables cortical circuits to encode sensory signals across diverse contexts. Somatostatin-expressing (SST) interneurons are well suited for this role through their strong recurrent connectivity with excitatory pyramidal cells. We tested the necessity of SST cells for inhibition stabilization in mouse primary visual cortex by selectively blocking excitatory glutamatergic receptors on SST cells. Antagonizing this key input for the recruitment of SST cells drives a paradoxical facilitation of their activity-the hallmark of inhibition stabilization-with increasing stimulus contrast, and even more so with high arousal. In a computational model of the visual cortex circuit, increasing sensory input and arousal both move the network toward a regime where other classes of interneurons are no longer sufficient for maintaining network stability. Thus, we reveal that the role of SST cells in cortical processing gradually switches as a function of both input strength and behavioral state.
Keywords: AMPA receptors; CP: Neuroscience; calcium imaging; chemogenetics; inhibition; microcircuit; normalization; pharmacology; visual cortex.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.R.T. and B.C.S. are on a patent application describing HTL.2 and its applications.
Figures




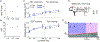
Update of
-
Behavioral state and stimulus strength regulate the role of somatostatin interneurons in stabilizing network activity.bioRxiv [Preprint]. 2024 Sep 10:2024.09.09.612138. doi: 10.1101/2024.09.09.612138. bioRxiv. 2024. Update in: Cell Rep. 2025 Jul 22;44(7):115954. doi: 10.1016/j.celrep.2025.115954. PMID: 39314375 Free PMC article. Updated. Preprint.
References
-
- Heeger DJ (1992). Normalization of cell responses in cat striate cortex. Vis. Neurosci 9, 181–197. - PubMed
-
- Palmigiano A, Fumarola F, Mossing DP, Kraynyukova N, Adesnik H, and Miller KD (2020). Common rules underlying optogenetic and behavioral modulation of responses in multi-cell-type V1 circuits. bioRxiv 11.11, 378729. 10.1101/2020.11.11.378729. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

