Out of the single-neuron straitjacket: Neurons within assemblies change selectivity and their reconfiguration underlies dynamic coding
- PMID: 40638616
- PMCID: PMC12320208
- DOI: 10.1113/JP288015
Out of the single-neuron straitjacket: Neurons within assemblies change selectivity and their reconfiguration underlies dynamic coding
Abstract
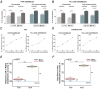
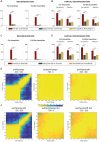
We investigated cell assemblies in the frontal cortex of macaques during two discrimination tasks. Focusing on the period of goal-action transformation, we extracted spikes fired during assembly activation from the full neural activity and showed that the contribution of a neuron to assembly coding, when it co-ordinates with other assembly neurons, differs from its coding in isolation. Neurons, with their flexible participation to multiple assemblies, contributed to the encoding of new information not encoded by the neurons alone. Even non-discriminative neurons acquired selectivity as part of the collective activity of the assemblies. Thus, neurons in their assemblies process distinct information for various purposes as a chess simul master, playing on multiple chessboards. The reconfiguration of the participation of the neurons into different assemblies in the goal-action transformation process translated into a dynamic form of coding, whereas minimal reconfiguration was associated with the static goal coding of the memory period. KEY POINTS: Traditionally, the coding properties of a neuron are studied using all its activity (full-spikes), irrespective of its co-ordination with different groups of neurons. With an assembly centered approach, we can determine the neuron's coding properties not in absolute terms, but relative to the assembly of neurons with which it co-ordinates. When neurons are studied in different assemblies-focusing only on the spikes fired during assembly coordination (assembly-spikes)-they can contribute to the coding of different variables. The coding flexibility of the same neuron in multiple assemblies increases the amount of information it can contribute to encoding compared to isolated neurons. Dynamic coding, as opposed to static coding, as observed during the goal-action transformation process, can be explained by an increase in the reconfiguration of active assemblies, with neurons contributing to the coding of different variables in different epochs, depending on which assembly is active.
Keywords: assembly; dynamic; monkey; prefrontal; reconfiguration; static.
© 2025 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
References
-
- Abbott, L. F. , & Dayan, P. (1999). The effect of correlated variability on the accuracy of a population code. Neural Computation, 11(1), 91–101. - PubMed
-
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B Methodology, 57(1), 289–300.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
