Metformin for patients with metastatic prostate cancer starting androgen deprivation therapy: a randomised phase 3 trial of the STAMPEDE platform protocol
- PMID: 40639383
- PMCID: PMC12303861
- DOI: 10.1016/S1470-2045(25)00231-1
Metformin for patients with metastatic prostate cancer starting androgen deprivation therapy: a randomised phase 3 trial of the STAMPEDE platform protocol
Abstract
Background: Metformin is a widely used anti-diabetic drug. Several studies have suggested that metformin has anticancer activity in some malignancies, including prostate cancer. Metformin might also mitigate the adverse metabolic effects of androgen-deprivation therapy (ADT). We hypothesised that metformin might improve survival in patients with metastatic hormone-sensitive prostate cancer and reduce metabolic complications associated with ADT.
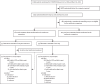
Methods: The STAMPEDE multi-arm, multi-stage, randomised phase 3 trial recruited patients with high-risk locally advanced or metastatic adenocarcinoma of the prostate staged by conventional imaging with isotope bone and CT scanning. This publication reports findings for the most recent STAMPEDE research question, testing the addition of metformin to standard of care for non-diabetic (glycated haemoglobin [HbA1c] <48 mmol/mol [equivalent to <6·5%]) patients with metastatic disease with adequate renal function (glomerular filtration rate ≥45 ml/min/1·73 m2) and WHO performance status 0-2. This trial recruited from 112 hospitals in the UK and Switzerland to the STAMPEDE protocol. Patients were randomly allocated (1:1) to standard of care or standard of care plus metformin 850 mg twice daily. Random assignment was by telephone using minimisation with a random element of 20% (developed and maintained by the MRC Clinical Trials Unit at UCL), stratified for randomising hospital, age (<70 years vs ≥70 years), WHO performance status (0 vs 1 or 2), type of ADT, regular long-term use of aspirin or non-steroidal anti-inflammatory drugs (NSAIDs; yes vs no), pelvic nodal status (positive vs negative), planned radiotherapy (yes vs no), and planned docetaxel or androgen receptor pathway inhibitor (ARPI) use (docetaxel vs abiraterone, enzalutamide, or apalutamide vs none). Standard of care comprised ADT with or without radiotherapy and with or without docetaxel or ARPI. The primary outcome measure was overall survival, defined as the time to death from any cause, assessed in the intention-to-treat population. Safety was assessed in patients who started treatment. The trial is registered with ClinicalTrials.gov, NCT00268476 and ISRCTN, ISRCTN78818544.
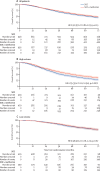
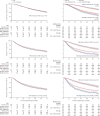
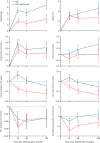
Findings: Between Sep 5, 2016, and Mar 31, 2023, 1874 patients with metastatic disease were randomly allocated to standard of care (n=938) or standard of care plus metformin (n=936). The median patient age was 69 years (IQR 63-73) and the median PSA was 84 ng/mL (24-352). 1758 (94%) of 1874 patients were newly diagnosed with metastatic disease and 116 (6%) were diagnosed with metachronous relapsing disease. 1543 (82%) of 1874 patients received ADT plus docetaxel and 52 (3%) received abiraterone, enzalutamide, or apalutamide. The median time to most recent case report form follow-up was 60 months (IQR 49-72). 473 deaths were reported in the standard of care group; median survival was 61·8 months (IQR 29·7 to not reached). There were 453 deaths in the metformin group; median survival was 67·4 months (32·5 to not reached; HR 0·91, 95% CI 0·80-1·03; p=0·15). Grade 3 or worse adverse events were reported in 487 (52%) of 938 patients in the standard of care group and 523 (57%) of 921 patients in the standard of care plus metformin group. 61 (7%) patients in the standard of care group and 84 (9%) patients in the standard of care plus metformin group reported at least one grade 3 or worse gastrointestinal adverse event; all other body systems showed no difference in grade 3 adverse events. There were six drug-related deaths in the standard of care group and one in the standard of care plus metformin group.
Interpretation: We did not find significant evidence of an overall survival benefit of adding metformin to standard of care in the overall population of patients with metastatic hormone-sensitive prostate cancer. The side-effect profile of metformin was as expected and consisted mainly of diarrhoea. Adverse metabolic side-effects of ADT were significantly reduced in the metformin group compared with the standard of care group.
Funding: Cancer Research UK, Prostate Cancer UK, and UK Research and Innovation Medical Research Council.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests SGi reports consulting fees from Tolremo, Ipsen, and Avalere Health; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Silvio Grasso Consulting, WebMD-Medscape, Peer Voice, European Society for Medical Oncology, Meister ConCept, Swiss Group for Clinical Cancer Research (SAKK), DESO, AdMeTech Foundation, EPG Health, and Intellisphere; support for attending meetings or travel from AstraZeneca, Bayer, Intellisphere, and Gilead; patents planned, issued, or pending for prostate cancer biomarkers (WO2009138392); participation on a data safety monitoring board or advisory board for Orion, Bayer, Astrazeneca, Myriad Genetic, Amgen, MSD, Bristol-Myers Squibb, Daiichi Sankyo, Boehringer Ingelheim, Innomedica, Macrogenics, Astellas, and Novartis; and leadership or fiduciary roles in other board, society, committee, or advocacy group, paid or unpaid for Pfizer, Unicancer, LinkinVax, University of Applied Sciences and Arts of Southern Switzerland, Advanced Prostate Cancer Consensus Conference Society, Fond'action, European Organisation for Research and Treatment of Cancer, American Society of Oncology. NDJ reports funding from Cancer Research UK and Prostate Cancer UK for trial conduct and translational substudies. AS reports support for the present manuscript from the Prostate Cancer Foundation-John Black Charitable Foundation Young Investigator Award; grants or contracts from the Prostate Cancer UK Research & Innovation Award, The Urology Foundation, and Cancer Research UK; consulting fees from Veracyte; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Ipsen, Janssen, and Gedeon Richter; and support for attending meetings or travel from AIRA Matrix. OE-T reports grants or contracts from The Royal College of Surgeons England. GA reports support for the present manuscript from Janssen, Pfizer, AstraZeneca, Astellas, Novartis, Arvinas, Bayer, Sanofi, Propella, and Orion; royalties or licenses from The Institute of Cancer Research Rewards to Discoverers Scheme; employment by UCL, which has out-licensing agreements with Veracyte and Artera that they could gain commercially from; patents planned, issued, or pending for blood-based methylation markers (GB1915469.9, issued); and other financial or non-financial interests from Janssen, Pfizer, AstraZeneca, Astellas, Novartis, Arvinas, Bayer, Sanofi, Propella, and Orion, during the conduct of the study. SC reports consulting fees from Janssen, Astellas, and Amgen and participation on a data safety monitoring board or advisory board for Janssen, Astellas, and Pharmaand. WC reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Astellas, Bayer, Janssen, Novartis AAA, and Ipsen and support for attending meetings or travel from Astellas, Bayer, Janssen, Novartis AAA, and Ipsen. DPD reports royalties or licenses from the Institute of Cancer Research; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Janssen; patents planned, issued, or pending issued for a localisation and stabilisation device (EP1933709B1); and participation on a data safety monitoring board or advisory board for Janssen. OD reports support for attending meetings or travel from Novartis. EG reports patents planned, issued, or pending for Decipher use as a predictive biomarker for docetaxel patent (filed). AH reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca, Janssen, Merck Serono, Novartis, and Pfizer; support for attending meetings or travel from Janssen and Merck Serono; and participation on a data safety monitoring board or advisory board for AstraZeneca, Janssen, and Pfizer. RJ reports grants or contracts from Astellas, Clovis, Exelixis, Bayer, and Roche; consulting fees from Janssen, Astellas, Bayer, Novartis, Pfizer, Merck Serono, MSD, Roche, Ipsen, and Bristol Myers Squibb; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Astellas, Janssen, Bayer, Pfizer, Merck Serono, MSD, Roche, Ipsen, Bristol Myers Squibb, and Merck Serono; support for attending meetings or travel from Bayer and Janssen; and participation on a data safety monitoring board or advisory board for Roche. REL reports support for the present manuscript from Medical Research Council Core Funding. OP reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AstraZeneca and Merck; support for attending meetings or travel from Astellas, Janssen, MSD, and RECORDATI; and participation on a data safety monitoring board or advisory board for Janssen. CP reports consulting fees from Blue Earth Therapeutics, Novartis, and Janssen and participation on a data safety monitoring board or advisory board for Telix Pharmaceuticals. SS reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Jannsen, Bayer UK and Astra Zeneca and support for attending meetings or travel from Jannsen, Bayer UK, and Merck. JST reports support for attending meetings or travel from Jansen, Roche, and Bayer and participation on a data safety monitoring board or advisory board for AstraZeneca, Astellas, and Bayer. FT reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Silvio Grasso Consulting, SAKK, Merck, and Novartis; support for attending meetings or travel from Bayer; and participation on a data safety monitoring board or advisory board for Bayer. MRS reports grants or contracts from Astellas, Janssen, and Sanofi-Aventis; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Eisai, Eli-Lily, and Janssen; support for attending meetings or travel from Health Research Board, Ireland, Trials Research Methodology Network, Ireland, and National Cancer Grid, India; and participation on a data safety monitoring board or advisory board for various academic sponsors (none paid). All other authors declare no competing interests.
Figures

References
-
- Tilki D, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. Part II—2024 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2024;86:164–182. - PubMed
-
- Dodkins J, Nossiter J, Cook A, et al. Does research from clinical trials in metastatic hormone-sensitive prostate cancer treatment translate into access to treatments for patients in the “real world”? A systematic review. Eur Urol Oncol. 2024;7:14–24. - PubMed
-
- Haseen F, Murray LJ, Cardwell CR, O'Sullivan JM, Cantwell MM. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4:128–139. - PubMed
-
- Jones C, Gray S, Brown M, et al. Risk of fractures and falls in men with advanced or metastatic prostate cancer receiving androgen deprivation therapy and treated with novel androgen receptor signalling inhibitors: a systematic review and meta-analysis of randomised controlled trials. Eur Urol Oncol. 2024;7:993–1004. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

