Acetylation of microtubule-binding PinX1 orchestrates ribosome biogenesis to nutrient starvation via the RNA polymerase I preinitiation complex
- PMID: 40639785
- PMCID: PMC12329599
- DOI: 10.1016/j.jbc.2025.110465
Acetylation of microtubule-binding PinX1 orchestrates ribosome biogenesis to nutrient starvation via the RNA polymerase I preinitiation complex
Abstract
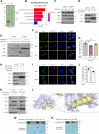
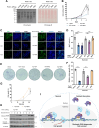
The shutdown of ribosome biogenesis is one of the sophisticated strategies for cells to save energy in response to nutrient starvation. However, the mechanism orchestrating ribosome biogenesis with cellular nutrition status remains unclear. Here, we identified the role of PIN2/TRF1-interacting telomerase inhibitor 1 (PinX1) in regulating ribosome biogenesis. PinX1 is highly expressed in colorectal cancers (CRC). Depletion of PinX1 impairs rDNA transcription, compromises ribosome biogenesis and inhibits tumor cells proliferation. Mechanically, associated with UBTF, PinX1 directly binds to RNA polymerase I subunit G (POLR1G) which is required for the assembly of RNA polymerase I preinitiation complex (PIC). Upon nutrient starvation, PinX1 is acetylated at K43, K133, K140, K149, K190, K222, which hinders its binding to POLR1G leading to disassembly of PIC. Collectively, our findings uncover a novel role of PinX1 and its acetylation, fine-tuning nucleolar transcription to stress signaling.
Keywords: POLR1G; PinX1; post-translational modification (PTM); ribosome biogenesis.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Focal adhesion kinase promotes ribosome biogenesis to drive advanced thyroid cancer cell growth and survival.Front Oncol. 2025 May 19;15:1252544. doi: 10.3389/fonc.2025.1252544. eCollection 2025. Front Oncol. 2025. PMID: 40458728 Free PMC article.
-
Ribosome biogenesis is a therapeutic vulnerability in pediatric neuroblastoma.Biochimie. 2025 Jul 21:S0300-9084(25)00159-2. doi: 10.1016/j.biochi.2025.07.018. Online ahead of print. Biochimie. 2025. PMID: 40701263
-
PINX1 inhibits proliferation and cisplatin resistance in nasopharyngeal carcinoma by promoting ILF3 ubiquitination.Am J Cancer Res. 2025 Jun 15;15(6):2518-2534. doi: 10.62347/WFER2605. eCollection 2025. Am J Cancer Res. 2025. PMID: 40667563 Free PMC article.
-
Nucleolar Organization in Response to Transcriptional Stress.Cancer Sci. 2025 Jul 29. doi: 10.1111/cas.70164. Online ahead of print. Cancer Sci. 2025. PMID: 40726293 Review.
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
References
-
- Mariño G., Pietrocola F., Eisenberg T., Kong Y., Malik S.A., Andryushkova A., et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol. Cell. 2014;53:710–725. - PubMed
LinkOut - more resources
Full Text Sources

