Pharmacological inhibition of Epac1 protects against pulmonary fibrosis by blocking FoxO3a neddylation
- PMID: 40639873
- PMCID: PMC12528777
- DOI: 10.1183/13993003.02250-2024
Pharmacological inhibition of Epac1 protects against pulmonary fibrosis by blocking FoxO3a neddylation
Abstract
Background: Idiopathic pulmonary fibrosis (IPF) is marked by progressive lung scarring with no existing cure, emphasising the need for new therapeutic targets. Current evidence suggests that cyclic adenosine monophosphate (cAMP) mitigates lung fibroblast proliferation via the protein kinase A pathway, but the impact of exchange proteins directly activated by cAMP 1 (Epac1) on IPF remains unexplored.
Objective: To investigate the role of Epac1 in IPF progression.
Methods: We examined lung samples from IPF patients and controls, and from a bleomycin-induced mouse model of pulmonary fibrosis. The effects of Epac were analysed in knockout mice and through modulation using viral vectors. The Epac1-specific small compound inhibitor AM-001 was evaluated in vitro using lung fibroblasts from patients with IPF, in vivo in bleomycin mice and ex vivo in IPF precision-cut lung slices.
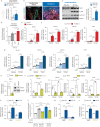
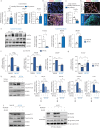
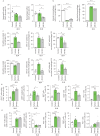
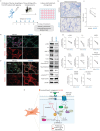
Results: Increased Epac1 expression was observed in lung tissues from IPF patients, fibrotic fibroblasts and bleomycin-challenged mice. Genetic or pharmacological inhibition of Epac1 with AM-001 decreased proliferation in normal and IPF fibroblasts, and reduced expression of profibrotic markers such as α-smooth muscle actin, transforming growth factor-β/SMAD family member 2/3, and interleukin-6/signal transducer and activator of transcription 3 pathways. Epac1-specific inhibition consistently protected against bleomycin-induced lung injury and fibrosis, suggesting significant therapeutic potential. Global gene expression profiling indicated a reduced profibrotic gene signature and neddylation pathway components in Epac1-deficient fibroblasts and human-derived lung cells. Mechanistically, the protective effects may involve inhibiting the neddylation pathway and preventing neural precursor cell expressed, developmentally downregulated 8 (NEDD8) activation, which in turn reduces the degradation of forkhead box protein O3 by NEDD8. Additionally, these effects may be enhanced while also limiting the proliferation of lung-infiltrating monocytes.
Conclusions: Our findings demonstrate that Epac1 regulates fibroblast activity in pulmonary fibrosis, and that targeting Epac1 with the pharmacological specific inhibitor AM-001 offers a promising therapeutic approach for treating IPF disease.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: F. Lezoualc'h is a co-founder and scientific advisor of Revadix SAS. All the other authors declare no affiliations with or involvement in any organisation or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Figures





Update of
-
Pharmacological Inhibition of Epac1 Protects against Pulmonary Fibrosis by Blocking FoxO3a Neddylation.bioRxiv [Preprint]. 2024 Sep 19:2024.09.13.612935. doi: 10.1101/2024.09.13.612935. bioRxiv. 2024. Update in: Eur Respir J. 2025 Oct 16;66(4):2402250. doi: 10.1183/13993003.02250-2024. PMID: 39345579 Free PMC article. Updated. Preprint.
Comment in
-
The cyclic AMP paradox in pulmonary fibrosis: context is everything….Eur Respir J. 2025 Oct 16;66(4):2501382. doi: 10.1183/13993003.01382-2025. Print 2025 Oct. Eur Respir J. 2025. PMID: 41101936 No abstract available.
References
-
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. doi: 10.1164/rccm.201308-1483ST - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
