Neutrophil-derived biomarkers in bronchiectasis: identifying a common therapeutic target
- PMID: 40639876
- PMCID: PMC12441583
- DOI: 10.1183/13993003.00081-2025
Neutrophil-derived biomarkers in bronchiectasis: identifying a common therapeutic target
Abstract
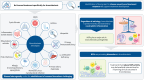
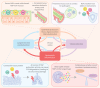
Bronchiectasis is a chronic respiratory disease that can lead to a substantial decline in lung function, ultimately leading to a significantly increased risk of morbidity and mortality. Despite the increasing global impact of bronchiectasis, no specific (or licensed) treatment for the disease currently exists, with most available therapies, though beneficial, focusing on symptom management and infection control. In part, the lack of specific treatments for bronchiectasis may be due to a lack of established biomarkers for the disease. Because bronchiectasis varies so widely in its clinical presentation and can be caused by various aetiologies, the establishment of validated biomarkers has proven challenging. However, identifying key biomarkers in bronchiectasis is crucial to developing appropriate diagnosis and management plans, as well as to measuring effective responses to treatment. While there is a multitude of potential biomarkers in bronchiectasis, almost all instances of bronchiectasis are underpinned by chronic neutrophilic inflammation. The imbalance in neutrophil serine proteases (NSPs) and their endogenous inhibitors has been strongly linked to the lung destruction, mucosal-related defects, infection and worsening of clinical outcomes that are frequently observed in bronchiectasis. In this review, we discuss the various biomarkers linked to bronchiectasis, with a specific focus on NSPs as the most validated biomarkers in bronchiectasis, given their marked role in the pathogenesis of the disease. Lastly, we touch on potential therapeutic approaches aimed at reducing NSP activity in bronchiectasis, showing that, to date, indirect NSP inhibition appears to be the strategy that most effectively addresses chronic neutrophilic inflammation in bronchiectasis.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: J.D. Chalmers reports support for the present publication from Boehringer Ingelheim, grants or contracts from AstraZeneca, Boehringer Ingelheim, Genentech, Gilead Sciences, GlaxoSmithKline, Grifols, Insmed, Novartis and Trudell Medical Group, consultancy fees from Antabio, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, GlaxoSmithKline, Grifols, Insmed, Janssen, Novartis, Pfizer, Trudell Medical Group and Zambon, and is the current Chief Editor of the European Respiratory Journal. M.A. Mall reports support for the present publication from Boehringer Ingelheim, grants or contracts from Boehringer Ingelheim, Enterprise Therapeutics, German Innovation Fund, German Ministry for Education and Research (BMBF), German Research Foundation (DFG) and Vertex Pharmaceuticals, with payments made to the institution, consultancy fees from Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Splisense and Vertex Pharmaceuticals, payment or honoraria for lectures from Vertex Pharmaceuticals, travel reimbursement received for participation in advisory board meetings for Boehringer Ingelheim and Vertex Pharmaceuticals, and fees for participation on an advisory board from Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Pari and Vertex Pharmaceuticals; M.A. Mall also reports that he is inventor on an issued patent filed by the University of North Carolina at Chapel Hill, describing the Scnn1b-transgenic mouse, and is an unpaid fellow of the European Respiratory Society. K.G. Nielsen reports support for the present manuscript from Boehringer Ingelheim and Nucleus Global, and has received honoraria for advisory boards/consulting from Boehringer Ingelheim, Ethris, Insmed, Parion Sciences and Recode Therapeutics. A.B. Chang reports support for the present manuscript from Boehringer Ingelheim and Nucleus Global, reports grants from the NHMRC and NHMRC-managed grants (Medical Research Futures Fund), Australia, is an independent data management committee member for clinical trials for Moderna (COVID-19, EBV and RSV vaccines), GlaxoSmithKline (an unlicensed vaccine) and AstraZeneca (monoclonal antibody), and has received fees to the institution for consulting on study designs for Boehringer Ingelheim and Zambon, airfares for travel from Boehringer Ingelheim and the ERS, and personal fees for being an author of two UpToDate chapters that are outside the submitted work. S. Aliberti reports support for the present manuscript from Boehringer Ingelheim, has received grants or contracts from GlaxoSmithKline, and reports consulting fees from AN2 Therapeutics, AstraZeneca, Boehringer Ingelheim, Brahms, Chiesi Farmaceutici, CSL Behring, Fondazione Internazionale Menarini, GlaxoSmithKline, Insmed, Menarini, Moderna, MSD Italia s.r.l, Pfizer, Physioassist, Verona Pharma, Vertex Pharmaceuticals and Zambon; S. Aliberti reports payments and/or honoraria from Boehringer Ingelheim, Fondazione Internazionale Menarini, Insmed, GlaxoSmithKline, Vertex Pharmaceuticals and Zambon, and has received payments for participating in an advisory/data safety monitoring board for AstraZeneca, Insmed, MSD Italia s.r.l and Verona Pharma. F. Blasi reports support for the present manuscript from Boehringer Ingelheim and Nucleus Global, has received grants from AstraZeneca, Chiesi Farmaceutici and Insmed, and has received consulting fees from Menarini; F. Blasi also reports payment/honoraria for lectures and advisory boards received from AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Grifols, Guidotti, Insmed, Menarini, Novartis, OM Pharma, Pfizer, Sanofi, Vertex Pharmaceuticals, Viatris and Zambon. B. Korkmaz reports support for the present manuscript from Boehringer Ingelheim and Nucleus Global, research contracts from Chiesi Farmaceutici, and grants from Boehringer Ingelheim and Insmed; B. Korkmaz has also been paid for the time spent as a committee member for advisory boards (Brensocatib Advisory Board (BRAB), Insmed), as well as for other forms of consulting (Boehringer Ingelheim, Neuprozyme Therapeutics Aps, Santhera Pharmaceuticals, Chiesi Farmaceutici, Gerson Lehrman Group), symposium organisation (Insmed), and travel support, lectures or presentations, outside the submitted work. N. Lorent reports support for the present manuscript from Boehringer Ingelheim and Nucleus Global, and has received honoraria payments to the institution for advisory boards/consulting and/or lectures from GlaxoSmithKline and Insmed, travel support from Pfizer, and is an unpaid member of the EMBARC Management Committee. C.C. Taggart reports support for the present manuscript from Boehringer Ingelheim and Nucleus Global, and grants from the Medical Research Council (MRC) and National Institute for Health and Care Research (NIHR), and has received funding from Chiesi Farmaceutici; C.C. Taggart also reports fulfilling a leadership or fiduciary role for Lung Research and Innovation Group, Asthma+Lung UK. M.R. Loebinger reports support for the present manuscript from Boehringer Ingelheim and Nucleus Global, and has received honoraria for advisory boards/consulting and/or lectures from 30T, AN2 Therapeutics, Armata, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici, Electromed, Ethris, Insmed, Mannkind, Parion Sciences, Recode Therapeutics and Zambon.
Figures