TLR-induced STK25 activation promotes IRF5-mediated inflammation
- PMID: 40639948
- PMCID: PMC12246392
- DOI: 10.26508/lsa.202503343
TLR-induced STK25 activation promotes IRF5-mediated inflammation
Abstract
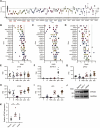
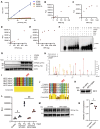
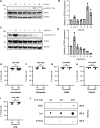
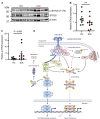
The transcription factor interferon regulatory factor 5 (IRF5) functions as an important mediator of the inflammatory response downstream of MyD88-dependent TLRs. Whereas dysregulation of IRF5 activity has been implicated in the development of numerous autoimmune diseases including systemic lupus erythematosus, the factors that modulate TLR-induced IRF5 post-translational modifications are poorly understood. The focus of this study was to identify novel kinases in TLR-MyD88-IRF5 signaling. We performed a kinome-wide siRNA screen in human THP-1 monocytic cells and identified serine/threonine protein kinase 25 (STK25) as a positive regulator of pro-inflammatory cytokine production via phosphorylation of IRF5 at Thr265, leading to IRF5 transcriptional activation. We further found that STK25 undergoes autophosphorylation in response to multiple TLR triggers. Findings were validated in Stk25-deficient primary immune cells revealing a significant attenuation in R848-induced IRF5 nuclear translocation and pro-inflammatory cytokine production. Finally, we detected increased levels of STK25 autophosphorylation in immune cells from systemic lupus erythematosus donors compared with healthy controls. These findings implicate STK25 as a new regulator of TLR7/8 signaling through the modulation of IRF5 activation.
© 2025 Rice et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
