Synaptic loss pattern is constrained by brain connectome and modulated by phosphorylated tau in Alzheimer's disease
- PMID: 40640137
- PMCID: PMC12246497
- DOI: 10.1038/s41467-025-61497-4
Synaptic loss pattern is constrained by brain connectome and modulated by phosphorylated tau in Alzheimer's disease
Abstract
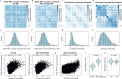
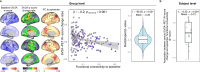
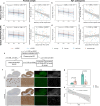
Synaptic loss strongly correlates with cognitive impairment in Alzheimer's disease (AD), yet the mechanism linking its origin and pattern remain unclear. Given that connected brain regions share molecular and synaptic features, and pathological tau, a key driver of synaptic degeneration, propagates through brain networks, we hypothesize that network architecture may influence synaptic loss in AD. By combining synaptic vesicle glycoprotein 2 A (SV2A) PET in 91 AD patients and 54 controls with normative connectome data, we show strongly connected regions exhibit similar levels of synaptic loss, and synaptic loss in one region is associated with connectivity-weighted synaptic loss in connected regions. Regions strongly connected to the epicenter show greater and faster synaptic loss. Plasma p-tau181 levels correlate with network-constrained synaptic loss, and post-mortem data confirm reduced SV2A expression in tau-rich areas. These findings support that synaptic vulnerability in AD is partially constrained by network topology and is modulated by phosphorylated tau.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Terry, R. D. et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol.: Off. J. Am. Neurological Assoc. Child Neurol. Soc.30, 572–580 (1991). - PubMed
-
- Finnema, S. J. et al. Imaging synaptic density in the living human brain. Sci. Transl. Med8, 348ra396 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

