Pattern recognition receptors: function, regulation and therapeutic potential
- PMID: 40640149
- PMCID: PMC12246121
- DOI: 10.1038/s41392-025-02264-1
Pattern recognition receptors: function, regulation and therapeutic potential
Abstract
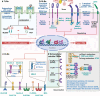
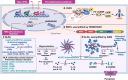
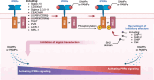
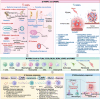
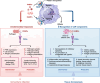
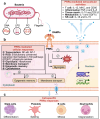
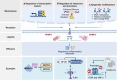
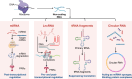
Pattern recognition receptors (PRRs) are sensors in the immune system, detecting pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). They serve as essential links between the innate and adaptive immune responses, initiating defense mechanisms against pathogens and maintaining immune homeostasis. This review examines the classification, structure, and signaling cascades of key PRR families, including toll-like receptors (TLRs), C-type lectin receptors (CLRs), nucleotide-binding oligomerization domain-like receptors (NLRs), AIM2-like receptors (ALRs), and others. It explores the dual roles of PRRs in immune defense and regulation, particularly through inhibitory PRRs (iPRRs), which prevent immune overactivation. The review also investigates the ligand recognition mechanisms and signaling pathways, highlighting the involvement of PRRs in disease progression and immune modulation. Notable signaling pathways, including NF-κB, MAPK, cGAS-STING, and MYD88-mediated and non-MYD88-mediated cascades, are discussed in the context of immune responses. Mechanisms that fine-tune PRR-mediated responses include transcriptional and fpost-transcriptional regulation, protein degradation, subcellular localization, and the recruitment of amplifiers and inhibitors, along with metabolic and microbial factors. These regulatory strategies ensure immune signaling remains adaptable and precise, preventing excessive inflammation. The review also explores the therapeutic potential of targeting PRRs in treating infectious, inflammatory, autoimmune, and malignant diseases, underscoring their importance in advancing immunological research and precision medicine.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
-
- Paul, E. Croonian lecture—on immunity with special reference to cell life. Proc. R. Soc. Lond.66, 424–448 (1900).
-
- Jenkins, M. K. & Schwartz, R. H. Pillars article: antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 165, 302–319 (1987). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

