PLIN2 promotes colorectal cancer progression through CD36-mediated epithelial-mesenchymal transition
- PMID: 40640171
- PMCID: PMC12246428
- DOI: 10.1038/s41419-025-07836-1
PLIN2 promotes colorectal cancer progression through CD36-mediated epithelial-mesenchymal transition
Abstract
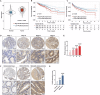
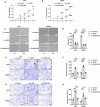
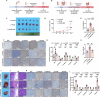
Colorectal cancer (CRC) is one of the most common malignant tumors with high incidence and mortality. The challenge remains to construct reliable prognostic prediction models and to further elucidate the key molecular mechanisms of tumor progression. To address this, we performed WGCNA based on 120 immune cell expression profiles from GEO sources to obtain a collection of monocytes/macrophages-related genes. The prognostic model was constructed by univariate survival analysis and LASSO regression analysis. Then, the prognostic model was validated by Multivariate Cox regression, Kaplan-Meier survival analysis and ROC analysis. In this prognostic model, we identified that PLIN2 has a potential value for CRC prognosis. PLIN2 expression in monocytes/macrophages was verified by scRNA-seq datasets and spatial transcriptome datasets, and PLIN2 was found to promote macrophage transformation to M2 subtype. Clinical specimens and tissue microarrays confirmed the differential expression and prognostic value of PLIN2 in CRC patients. Functional experiments demonstrated that PLIN2 gene overexpression promoted the proliferation, migration and invasion of CRC cells and significantly facilitated tumor growth in vivo. Mechanistically, we revealed that CD36 is a potential downstream target gene of PLIN2. The CD36 inhibitor Sulfo-N-succinimidyl Oleate significantly reversed PLIN2-induced proliferation, migration, invasion, and EMT activity of CRC cells in vitro and in vivo. Immunoprecipitation and immunofluorescence experiments confirmed that PLIN2 could interact with CD36. PLIN2 stabilized CD36 protein expression by inhibiting the proteasomal degradation pathway, thereby promoting CD36-mediated EMT activity. Overall, our study highlights that the PLIN2/CD36 axis regulates EMT activity and CRC progression, suggesting that interventions in this signaling pathway may offer a promising therapeutic approach to CRC progression. Schematic diagram elucidating the role of PLIN2 in CRC by Figdraw. FA is transported into the cell via CD36-mediated endocytosis. In CRC cells, PLIN2 promotes stability of CD36 and interacts with CD36 to activate the EMT process. However, the CD36 inhibitor SSO inhibits the binding of FAs to CD36 and attenuates its endocytosis, thereby reversing the PLIN2-mediated EMT process. Ultimately, the PLIN2-induced enhancement of CRC cell proliferation, migration, and invasion is attenuated by the CD36 inhibitor SSO.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: All clinical specimens were obtained from participants who provided written informed consent, and the study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (approval number: 2408-Exp059). All animal protocols were approved by the Experimental Animal Ethics Committee of Shanghai SINOGENE Life Technology Co., Ltd (approval number: XNG201-2407-001). All methods were performed in accordance with the relevant guidelines and regulations, including the principles outlined in the Declaration of Helsinki for human research and the Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press) for animal studies.
Figures






References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clinicians. 2024;74:229–63. - PubMed
-
- Prager GW, Taieb J, Fakih M, Ciardiello F, Van Cutsem E, Elez E, et al. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N Engl J Med. 2023;388:1657–67. - PubMed
-
- Kelly ME, Spolverato G, Lê GN, Mavros MN, Doyle F, Pawlik TM, et al. Synchronous colorectal liver metastasis: A network meta-analysis review comparing classical, combined, and liver-first surgical strategies. Journal Surgical Oncol. 2015;111:341–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

