Advancing equitable access to innovation in breast cancer
- PMID: 40640192
- PMCID: PMC12246153
- DOI: 10.1038/s41523-025-00768-1
Advancing equitable access to innovation in breast cancer
Erratum in
-
Author Correction: Advancing equitable access to innovation in breast cancer.NPJ Breast Cancer. 2025 Dec 16;11(1):140. doi: 10.1038/s41523-025-00871-3. NPJ Breast Cancer. 2025. PMID: 41402330 Free PMC article. No abstract available.
Abstract
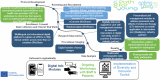
This manuscript critically examines the challenges associated with the design and conduct of academic global breast cancer trials outside the influence of pharmaceutical companies, leveraging insights from the Breast International Group (BIG). In the past 4 decades significant declines in breast cancer mortality have occurred, partly related to industry-academic clinical and translational partnerships with long term study follow up. However, in the past decade these partnerships have largely uncoupled. The increasing complexity and non-alignment of trials, funding constraints, regulatory complexity, declining academic freedom, lack of transparency, and lack of affordability of new agents have become key barriers to equitably improving cancer outcomes. Industry research expenditure in the United States is now 5 fold greater than publically funded academic research. To address these challenges, we advocate for patient centred systemic reforms, with trials balancing commercial interests with public health imperatives. These reforms should include equitable research funding models, streamlined international clinical trial regulatory processes, and increased collaboration across diverse stakeholders. Practical solutions to enhance global trial accessibility and efficacy include leveraging digital technologies, artificial intelligence, real world data, decentralizing clinical trial infrastructure, and embedding translational research frameworks across countries.
Conflict of interest statement
Competing interests: Conflict of Interest Travel: Servier (ER), Gilead (ER, EB) Astra Zeneca (ER, EB, GC) Integris (ER), Pfizer (ER, EB), Genesis Pharma (ER), Bristol Myers Squibb (ER), Roche (ER, GC), Daichi Sankyo (EB, GC, SOR), Exact Sciences (EB), Novartis (EB,IVL, SOR), Merck (SOR), Nordic Pharma (SOR)Stocks: Eli Lilly (EC)Honoraria/Lecture Fees: Chugai (SS), Kyowa Kirin (SS), Merck Sharp Dohme (SS, GW), Takeda (SS, EB), Novartis (SS, GW, ER, EB, GC), Daichi Sankyo (SS, GW, EB,GC,BL), Eisai (SS), Eli Lilly (SS, EB, GC), Astra Zeneca (SS, GW, ER, EB, GC), Pfizer (EB, GC, GW,SS), Taiho (SS), Oho (SS), Nippon Kayaku (SS), Gilead (SS, ER), Exact Sciences (SS, GC,EB), Myriad Genetics (SS), Roche (GW,GC), Ser-vier (ER), Foundation Medicine (GC), Samsung (GC), Incyte (EB), Seagen (GC), Menarini (GC), Gilead Sciences (GC), Ellipses Pharma (GC).Consultancy/Advisory RolesGlaxo Smith Kline (GC), Zymeworks (PB), Astra Zeneca (GW,GC, BL, SL), Roche (SL, GC), Janssen (PB), Repare (PB), Eli Lilly (GC, SL, BL,PB), Exact Sciences (SL,EB), Gilead Sciences (GC, SL, BL), Amgen (PB), Foundation Medicine (GC), Bristol Myers Squibb (GC,SL), Samsung (GC), Boehringer Ingelheim (GC), Merck (GC, PB), Blueprint Medicines (GC), Sandoz (EB), Pfizer (EB, GC, BL), Menarini (EB, GC, BL), Exnet Science (GC), Daichi Sankyo (GW, EB, BL), Novartis (GW, GC,SL), Merck Sharp Dohme (GW), Seagen (GC,PB), Guardant Health (GC), Celulicity (GC), Hengrini Therapeutics (GC), Amaroq Therpeutics (SL), Mersana Therpeutics (SL), Domain Therpeutics (SL), BioNTech (SL), Menarini Asia Pacific (SL), Bicycle Therpeutics (SL), Saga Diagnostics (SL), Adanate (SL), Research Funding to InstitutionAmgen (PB, IVL), Signal Chem (PB) Seagen (PB), Servier (PB), Sanofi Aventis (SS, PB, AA, VA, TG, CS), Janssen (JB, GW), Clovis (JB), Bristol Myers Squibb (SL, PB), Lilly Loxo (PB), Medice-na (PB), PTC Thereputics (PB), Life Sciences (PB), Zymeworks (PB), Veracyte (BC, PB), Taiho (SS), Eisai (SS), Chugai (SS), Medimmune (GW), Novartis (IVL, SL, DC, GW, EC, AA, VA, TG, CS, JB, PB), Pfizer (DC, SL, GW, EC, AA, VA, CS, TG, JB), Merck Sharp Doehm (SL,JB,SS), Takeda (SS, GW), Astra Zeneca (SL, DC, SS, PB, GW, EC, BL, IVL), Daichi Sankyo (DC,SS), Roche (SL, DC, GW, EC, AA, VA, CS, TG), Merck (GW, GC,PB), Bayer (GW), Astellas (GW), PUMA biotech (JB), Gilead (SL, SS, GC, PB), Eli Lilly (SS, SL, EC, PB), Glaxo Smith Kline (GW, PB, AA, VA, TG, CS), Libos (GW), Boehringer Ingelheim (PB), Greenwich Lifesciences (EC), Nektar (PB), Exact Sciences (DC), Ely Lilly (DC), Menarini (DC), Resilience Care (IVL), Pfizer Edimark (IVL), Sandoz (IVL), Chugai (IVL), Servier Monde (IVL),Doctaform Medical Market-ing (IVL), Nektar Therapeutics (SL),No Conflicts to DeclareAU.
Figures


References
-
- Piccart-Gebhart M. J., Goulioti T., Straehle C., Cameron D. Overcoming barriers to clinical trials cooperation: the Breast International Group example. 2021 ASCO Educational Handbook. 231-239 10.1200/EDBK_321475. - PubMed
-
- DeSantis, C. E. et al. Cancer statistics for African Americans. CA Cancer J. Clin.69, 211–233 (2019). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

