Induction of systemic and mucosal immune response against Zika virus by vaccination with non-infectious chimeric VLPs
- PMID: 40640219
- PMCID: PMC12246235
- DOI: 10.1038/s41598-025-07059-6
Induction of systemic and mucosal immune response against Zika virus by vaccination with non-infectious chimeric VLPs
Abstract
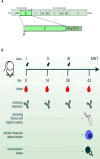
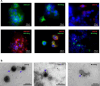
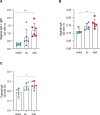
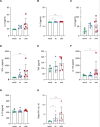
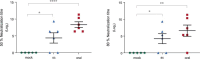
The Zika virus (ZIKV) causes acute febrile illness and can lead to complications such as Guillain-Barré syndrome and congenital disorders. As arbovirus outbreaks increase, vaccination becomes a crucial preventive strategy. Currently, no commercial vaccines are available for ZIKV, which is transmitted by mosquitoes and bodily fluids, underscoring the need for a safe vaccine that induces both systemic and mucosal immune responses. In this study, we present a ZIKV vaccine candidate utilizing virus-like particles (VLPs) technology combined with variant-specific surface proteins (VSP) from Giardia lamblia. Previous research demonstrated that these VSP act as effective adjuvants and are resistant to gastrointestinal degradation, expanding administration possibilities via orogastric routes in addition to the conventional subcutaneous route. To develop the immunogen, we engineered retrovirus-derived VLPs decorated with the ZIKV envelope glycoprotein (ZIKV-E) as the target antigen, incorporating VSPs on their surface. Immunocompetent Balb/c mice were immunized with VSP-VLPs ZIKV-E via oral and subcutaneous routes. Immune characterization revealed robust systemic and mucosal humoral responses, as well as a specific cellular activation. Moreover, a significant neutralizing capacity of serum antibodies was observed. These findings highlight the potential of the vaccine candidate to elicit a targeted immune response, achieved through different administration methods.
Keywords: Arbovirus; Mucosal response; Subunit vaccines; Zika virus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics statement: This research strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The well-being of all animals and the management of animal facilities were supervised by CICUAL-UCC. The study protocol was approved by CICUAL-UCC, guaranteeing adherence to ethical guidelines. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Vesicular Stomatitis Virus and DNA Vaccines Expressing Zika Virus Nonstructural Protein 1 Induce Substantial but Not Sterilizing Protection against Zika Virus Infection.J Virol. 2020 Aug 17;94(17):e00048-20. doi: 10.1128/JVI.00048-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32554698 Free PMC article.
-
BALB/c mice immunized with a combination of virus-like particles incorporating Kaposi sarcoma-associated herpesvirus (KSHV) envelope glycoproteins gpK8.1, gB, and gH/gL induced comparable serum neutralizing antibody activity to UV-inactivated KSHV.Oncotarget. 2017 May 23;8(21):34481-34497. doi: 10.18632/oncotarget.15605. Oncotarget. 2017. PMID: 28404899 Free PMC article.
-
Manipulation of immunodominant variable epitopes of norovirus capsid protein elicited cross-blocking antibodies to different GII.4 variants despite the low potency of the polyclonal sera.J Virol. 2025 Jul 22;99(7):e0061125. doi: 10.1128/jvi.00611-25. Epub 2025 May 30. J Virol. 2025. PMID: 40444946 Free PMC article.
-
Congenital Zika syndrome: Pitfalls in the placental barrier.Rev Med Virol. 2018 Sep;28(5):e1985. doi: 10.1002/rmv.1985. Epub 2018 May 15. Rev Med Virol. 2018. PMID: 29761581
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
References
-
- Encefalitis equina del oeste - Argentina. https://www.who.int/es/emergencies/disease-outbreak-news/item/2023-DON499.
MeSH terms
Substances
Grants and funding
- PICT-2019-00420/Agency for Promotion of Science and Technology of Argentina
- PICT-2019-00420/Agency for Promotion of Science and Technology of Argentina
- PICT-2019-00420/Agency for Promotion of Science and Technology of Argentina
- PICT-2019-00420/Agency for Promotion of Science and Technology of Argentina
- 80020220300055CC/Catholic University of Córdoba
- 80020220300055CC/Catholic University of Córdoba
- 80020220300055CC/Catholic University of Córdoba
- 80020220300055CC/Catholic University of Córdoba
- PIP 2017-2019 GI. 11220170100731CO/National Council for Scientific and Technical Research of Argentina (CONICET)
- PIP 2017-2019 GI. 11220170100731CO/National Council for Scientific and Technical Research of Argentina (CONICET)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

