DSP and DPP are dispensable for initiation of dentin and enamel mineralization but critical for circumpulpal dentin mineralization
- PMID: 40640300
- PMCID: PMC12246090
- DOI: 10.1038/s41598-025-09743-z
DSP and DPP are dispensable for initiation of dentin and enamel mineralization but critical for circumpulpal dentin mineralization
Abstract
Dentin sialophosprotein (DSPP) is cleaved into the N-terminal dentin sialoprotein (DSP) proteoglycan and the C-terminal dentin phosphoprotein (DPP; the most acidic proteins in humans). To define the functions of DSP and DPP, we generated a Dspp-DPP mouse model using CRISPR/Cas9 technology and compared tooth mineralization in Dspp-DPP and Dspp-/- mice in the C57BL/6 background. In both mice, the initiation of dentin mineralization was associated with matrix vesicles. Odontoblasts appeared normal with odontoblastic processes. In Dspp-/- mice, limited mineralized dentin was observed. In Dspp-DPP/-DPP mice, dentin globules (calcospherites) with varied mineral density and unmineralized interglobular dentin were observed throughout the circumpulpal dentin. The area of predentin was smaller compared to Dspp-/- mice, but larger than wild-type mice. In Dspp-/- and Dspp-DPP/-DPP mice, enamel formation was comparable to wild-type. In both mice, Dmp1 expression in differentiating and differentiated odontoblasts was altered. We propose a model for dentin mineralization in which DSP, enriched in the peritubular dentin, propagates mineralization within the hypomineralized calcospherites in the intertubular dentin while DPP is essential for the maturation of calcospherite mineralization in the circumpulpal dentin. We conclude that DSP and DPP are dispensable for the initiation of dentin and enamel mineralization, but critical for circumpulpal dentin mineralization.
Keywords: Biomineralization; Dentin; Dentin Sialophosphoprotein; Dentin mineralization; Dentin phosphoprotein; Dentin sialoprotein.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Conflict of interest: The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a conflict of interest.
Figures






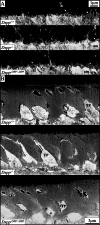


Similar articles
-
Functions of secretory calcium-binding phosphoproteins in dental mineralization.J Bone Miner Res. 2025 Jul 28;40(8):909-930. doi: 10.1093/jbmr/zjaf062. J Bone Miner Res. 2025. PMID: 40318225 Review.
-
Role of the NH2 -terminal fragment of dentin sialophosphoprotein in dentinogenesis.Eur J Oral Sci. 2013 Apr;121(2):76-85. doi: 10.1111/eos.12020. Epub 2013 Feb 7. Eur J Oral Sci. 2013. PMID: 23489896 Free PMC article.
-
Dentin defects caused by a Dspp-1 frameshift mutation are associated with the activation of autophagy.Sci Rep. 2023 Apr 19;13(1):6393. doi: 10.1038/s41598-023-33362-1. Sci Rep. 2023. PMID: 37076504 Free PMC article.
-
Dentin sialophosphoprotein in biomineralization.Connect Tissue Res. 2010 Oct;51(5):404-17. doi: 10.3109/03008200903329789. Connect Tissue Res. 2010. PMID: 20367116 Free PMC article. Review.
-
Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization.Matrix Biol. 2009 May;28(4):221-9. doi: 10.1016/j.matbio.2009.03.006. Epub 2009 Apr 5. Matrix Biol. 2009. PMID: 19348940 Free PMC article.
References
-
- Weiner, S., Addadi, L. & Biomineralization At the cutting edge. Science298, 375–376. 10.1126/science.1078093 (2002). - PubMed
-
- Knoll, A. H. Biomineralization and evolutionary history. Rev. Mineral. Geochem.54, 329–356 (2003).
-
- Smith, C. E. Cellular and chemical events during enamel maturation. Crit. Reviews Oral Biology Medicine: Official Publication Am. Association Oral Biologists. 9, 128–161. 10.1177/10454411980090020101 (1998). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

