Synthesis of thiosemicarbazone Schiff base derivatives as anti-leishmanial agents and molecular dynamics simulations insights
- PMID: 40640503
- PMCID: PMC12246158
- DOI: 10.1038/s41598-025-10545-6
Synthesis of thiosemicarbazone Schiff base derivatives as anti-leishmanial agents and molecular dynamics simulations insights
Abstract
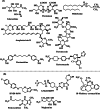
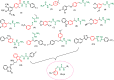
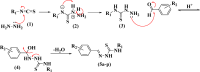
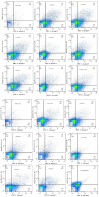
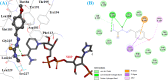
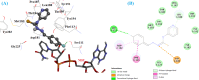
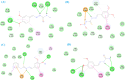
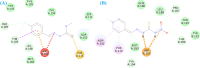
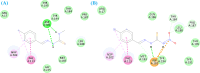
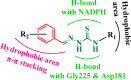
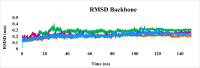
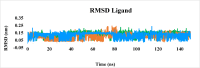
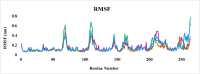
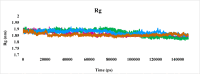
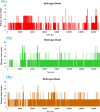
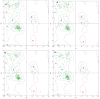
Leishmaniasis is one of the infectious diseases caused by protozoa and is considered the second most significant parasitic disease after malaria. In this research, thiosemicarbazone Schiff base derivatives were synthesized via a one-pot, two-step, three-component reaction. Then, the effectiveness of the compounds against the two forms of Leishmania major and Leishmania tropica called amastigote and promastigote, was tested. All synthesized compounds displayed weak to good anti-amastigote and anti-promastigote activities. Notably, compounds 5g and 5p were the most potent compounds against amastigote and promastigote forms, respectively, of Leishmania major, with IC50 = 26.7 µM and 12.77 µM. Analogues 5e and 5g were the most potent compounds against amastigote and promastigote forms of Leishmania tropica, with IC50 = 92.3 µM and 12.77 µM, respectively. The cytotoxicity activity of the compounds was also evaluated using the J774.A1 cell lines. Some of the screened derivatives displayed low cytotoxicity to macrophage infection. Among compounds, 5p and 5e showed the highest SI (95.4 and 34.6) against L. major and L. tropica, respectively. In the next phase, the most effective thiosemicarbazone derivatives were examined for their ability to induce apoptosis in promastigotes. According to the results, these compounds displayed different early and late apoptosis as well as necrotic effects on promastigotes of Leishmania major and Leishmania tropica. Furthermore, the compounds' drug-likeness and pharmacokinetic parameters were predicted in silico. All compounds showed acceptable drug-likeness and pharmacokinetic profiles. Furthermore, the most likely sites of the compounds metabolized by the major cytochromes were identified. Additionally, the in silico compounds' cardiotoxicity potential was assessed. This investigation showed compounds 5m-p were cardiotoxic. Lastly, molecular docking and molecular dynamics simulations were performed to explore the potential mechanisms of anti-leishmanial activity in the LmPRT1 active site.
Keywords: Amastigote; Apoptosis; Hydrazine-1-carbothioamide; Promastigote; Tropical disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
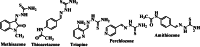
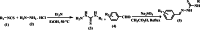



References
-
- Gupta, O., Pradhan, T., Bhatia, R. & Monga, V. Recent advancements in anti-leishmanial research: synthetic strategies and structural activity relationships. Eur. J. Med. Chem.223, 113606. 10.1016/j.ejmech.2021.113606 (2021). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

