De novo assembly and delivery of synthetic megabase-scale human DNA into mouse early embryos
- PMID: 40640530
- PMCID: PMC12328230
- DOI: 10.1038/s41592-025-02746-8
De novo assembly and delivery of synthetic megabase-scale human DNA into mouse early embryos
Abstract
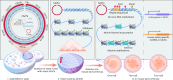
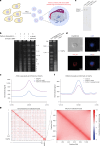
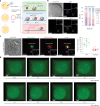
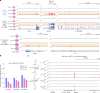
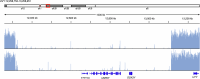
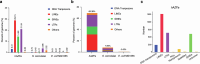
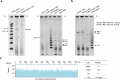
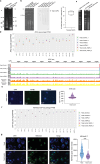
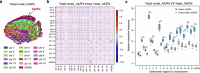
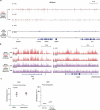
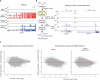
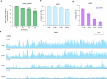
Epigenetic modifications on natural chromosomes are inherited and maintained in a default state, making it challenging to remove intrinsic marks to study the fundamental principles of their establishment and further influence on transcriptional regulation. In this study, we developed SynNICE, a method for assembling and delivering intact, naive, synthetic megabase (Mb)-scale human DNA into early mouse embryos, to study de novo epigenetic regulation. By assembling and delivering a 1.14-Mb human AZFa (hAZFa) locus, we observed the spontaneous incorporation of murine histones and the establishment of DNA methylation at the one-cell stage. Notably, DNA methylation from scratch strongly enriches at repeat sequences without H3K9me3 reinforcement. Furthermore, the transcription of hAZFa initiated at the four-cell stage is regulated by newly established DNA methylation. This method provides a unique platform for exploring de novo epigenomic regulation mechanisms in higher animals.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.Y., Y.L., J.Z. and Z.L. are co-investors on three patents related to SynNICE. L.Y. is co-founder and CEO of Qihan Biotech. The other authors declare no competing interests.
Figures




Similar articles
-
Stage-specific DNA methylation dynamics in mammalian heart development.Epigenomics. 2025 Apr;17(5):359-371. doi: 10.1080/17501911.2025.2467024. Epub 2025 Feb 21. Epigenomics. 2025. PMID: 39980349 Review.
-
[Epigenetics' implication in autism spectrum disorders: A review].Encephale. 2017 Aug;43(4):374-381. doi: 10.1016/j.encep.2016.07.007. Epub 2016 Sep 28. Encephale. 2017. PMID: 27692350 French.
-
Novel application of metabolic imaging of early embryos using a light-sheet on-a-chip device: a proof-of-concept study.Hum Reprod. 2025 Jan 1;40(1):41-55. doi: 10.1093/humrep/deae249. Hum Reprod. 2025. PMID: 39521726 Free PMC article.
-
Epigenetic timing effects on child developmental outcomes: a longitudinal meta-regression of findings from the Pregnancy And Childhood Epigenetics Consortium.Genome Med. 2025 Apr 14;17(1):39. doi: 10.1186/s13073-025-01451-7. Genome Med. 2025. PMID: 40229801 Free PMC article.
-
High-throughput library transgenesis in Caenorhabditis elegans via Transgenic Arrays Resulting in Diversity of Integrated Sequences (TARDIS).Elife. 2023 Jul 4;12:RP84831. doi: 10.7554/eLife.84831. Elife. 2023. PMID: 37401921 Free PMC article.
References
-
- Cello, J., Paul, A. V. & Wimmer, E. Chemical synthesis of poliovirus cDNA: generation of infectious virus in the absence of natural template. Science297, 1016–1018 (2002). - PubMed
-
- Gibson, D. G. et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science329, 52–56 (2010). - PubMed
-
- Richardson, S. M. et al. Design of a synthetic yeast genome. Science355, 1040–1044 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

