SFX-01 is therapeutic against myeloproliferative disorders caused by activating mutations in Shp2
- PMID: 40640547
- PMCID: PMC12340136
- DOI: 10.1038/s44321-025-00267-7
SFX-01 is therapeutic against myeloproliferative disorders caused by activating mutations in Shp2
Abstract
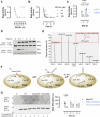
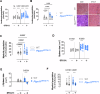
Activating mutations of Src homology-2 domain-containing protein tyrosine phosphatase-2 (Shp2) cause multiple childhood conditions for which there is an unmet therapeutic need, including juvenile myelomonocytic leukemia (JMML) and Noonan syndrome. SFX-01, an α-cyclodextrin-stabilized sulforaphane complex currently in clinical development, covalently adducts cysteine residues. Using unbiased proteomics, its protein targets were identified, including Shp2. SFX-01 induced an inhibitory dithiolethione modification at the Shp2 active site cysteine. Importantly, in a transgenic mouse model of human Noonan syndrome with hyperactive D61G Shp2, SFX-01 concomitantly normalized their phosphatase activity and myeloid cell count. Furthermore, SFX-01 also attenuated JMML human patient-derived hematopoietic stem cell proliferation that was linked to STAT1 signaling and decreased cyclin D1 expression, resulting in cell-cycle arrest. We conclude that SFX-01 is an activating mutant Shp2 inhibitor and may offer beneficial effects in patients with JMML or Noonan syndrome.
Keywords: Myeloproliferative Disorders; Noonan Syndrome; SFX-01; Shp2; Sulforaphane.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests. PE acknowledges he was an unpaid advisor to Theracryf plc.
Figures




References
-
- Alpha-Tocopherol BCCPSG (1994) The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330:1029–1035 - PubMed
-
- Araki T, Mohi MG, Ismat FA, Bronson RT, Williams IR, Kutok JL, Yang W, Pao LI, Gilliland DG, Epstein JA et al (2004) Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med 10:849–857 - PubMed
-
- Artoni A, Selicorni A, Passamonti SM, Lecchi A, Bucciarelli P, Cerutti M, Cianci P, Gianniello F, Martinelli I (2014) Hemostatic abnormalities in Noonan syndrome. Pediatrics 133:e1299–1304 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

