Maternal stressors disrupt mouse placental proteome and fetal brain development in a sex-specific fashion through inflammation and oxidative stress
- PMID: 40640553
- PMCID: PMC12532718
- DOI: 10.1038/s41380-025-03090-1
Maternal stressors disrupt mouse placental proteome and fetal brain development in a sex-specific fashion through inflammation and oxidative stress
Abstract
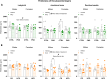
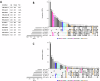
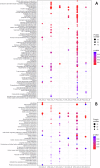
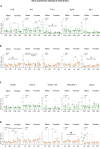
Adverse maternal conditions during pregnancy result in an increased risk for neuropsychiatric disorders in the offspring, although the underlying mechanisms are poorly understood. We have recently shown that two distinct insults, prenatal stress (PNS) or maternal high-fat diet (mHFD), increase inflammation and oxidative stress in the brain of adolescent female mice. Here, we sought to investigate the early mechanisms underlying such effects, focusing on the placenta and fetal brain, as well as the protective effects of the antioxidant N-acetyl-cysteine (NAC), in C57Bl6/N mice. We used a multi-disciplinary approach combining proteomic, metabolomic, lipidomic and histological analysis to characterize the structural and functional changes of the placenta; moreover, a targeted gene expression analysis was carried out in the brains of male and female fetuses to evaluate oxidative stress and inflammatory-related changes. Our data highlight comparable, but sex-specific, responses to the two maternal stressors, which target placenta and fetal brain, and are buffered by NAC administration. Placental function was specifically disrupted in males, with signaling pathways of cardio-metabolic risk emerging in this sex. By contrast, fetal brain was affected in females, with an increased expression of genes related to inflammation and oxidative stress. In conclusion, we provide evidence for an early origin of sex-dependent embedding of prenatal adverse experiences in different organs which might explain differential susceptibility to later disease trajectories.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Cirulli F, Musillo C, Berry A. Maternal obesity as a risk factor for brain development and mental health in the offspring. Neuroscience. 2020;447:122–35. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

