Mesenchymal stem cell-secreted KGF ameliorates acute lung injury via the Gab1/ERK/NF-κB signaling axis
- PMID: 40640718
- PMCID: PMC12243209
- DOI: 10.1186/s11658-025-00757-z
Mesenchymal stem cell-secreted KGF ameliorates acute lung injury via the Gab1/ERK/NF-κB signaling axis
Abstract
Background: The epithelial sodium channel (ENaC) situated in the apical membrane of alveolar epithelial type 2 (AT2) cells is beneficial to edematous fluid reabsorption in acute lung injury (ALI). Recently, mesenchymal stem cells (MSCs), particularly their secretome, has emerged as a novel approach for treating pulmonary diseases. Among these secreted factors, keratinocyte growth factor (KGF) plays a critical role in mediating alveolar epithelial repair during ALI by enhancing epithelial cell proliferation, restoring epithelial integrity, and alleviating pulmonary edema, making it a promising candidate for therapeutic strategies. This study primarily focused on investigating the impact of KGF secreted from MSC on ALI, and clarifying its specific mechanism in regulating the expression of ENaC.
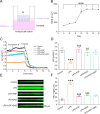
Methods: Lipopolysaccharide (LPS)-stimulated primary mouse AT2 cells were treated with KGF in vitro, and western blots along with immunofluorescence assays were performed to investigate the regulatory mechanism of KGF on ENaC protein expression. To further confirm the role of mouse bone marrow MSC-derived KGF, co-culture experiments with AT2 cells and either MSC or MSC with KGF knockdown (MSC-siKGF) were conducted. In vivo, an ALI model was established in mice by LPS-induced lung injury. The therapeutic effects of tail vein-injected MSC or MSC-siKGF were assessed using hematoxylin-eosin staining, lung wet/dry weight ratio, and alveolar fluid clearance.
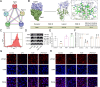
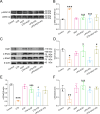
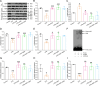
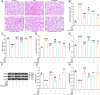
Results: In primary mouse AT2 cells, KGF stimulation effectively restored the reduction of growth factor receptor-bound protein 2-associated binding protein 1 (Gab1) and α/γ-ENaC protein levels induced by LPS. KGF inhibited the activation of the LPS-induced extracellular regulated protein kinases (ERK) and nuclear factor-kappaB (NF-κB) signaling pathway. Treatment with the ERK pathway inhibitor PD98059 reversed the LPS-induced reduction in ENaC protein levels but had no effect on Gab1 levels. In addition, PD98059 suppressed LPS-induced activation of the NF-κB signaling pathway. Further analysis revealed that LPS stimulation weakened the interaction between the NF-κB p65 subunit and inhibitor kappaB (IκB), while KGF enhanced this interaction and inhibited the nuclear translocation of p65. Both KGF and the NF-κB inhibitor QNZ reversed the LPS-induced downregulation of ENaC protein levels and gene expression. Furthermore, both agents effectively restored the functional activity of ENaC channels. Co-culture with MSCs increased Gab1 protein levels, inhibited ERK/NF-κB signaling activation, and suppressed p65 nuclear translocation in LPS-treated AT2 cells, whereas these effects were attenuated in cells co-cultured with MSC-siKGF. In an ALI mouse model, tail-vein injection of MSCs alleviated lung injury and pulmonary edema, while the therapeutic effects of MSC-siKGF were weaker they were partly restored by the combination of QNZ.
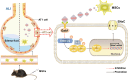
Conclusions: Our study validated that the efficacy of MSCs in the treatment of edematous ALI was significantly associated with KGF, which potentially enhanced the upregulation of ENaC through the Gab1/ERK/NF-κB signaling pathway.
Keywords: Acute lung injury; Epithelial sodium channel; Keratinocyte growth factor; Mesenchymal stem cell; Nuclear factor-kappaB.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal studies complied with the ethical guidelines for researchers by the International Council for Laboratory Animal Science (ICLAS) and were approved by the China Medical University Animal Care Committee (permission number: KT2021041; date issued: 23 February 2021). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Mesenchymal stem cell conditioned medium alleviates acute lung injury through KGF-mediated regulation of epithelial sodium channels.Biomed Pharmacother. 2023 Dec 31;169:115896. doi: 10.1016/j.biopha.2023.115896. Epub 2023 Nov 18. Biomed Pharmacother. 2023. PMID: 37984305
-
Endothelial cell-derived extracellular vesicles modulate the therapeutic efficacy of mesenchymal stem cells through IDH2/TET pathway in ARDS.Cell Commun Signal. 2024 May 27;22(1):293. doi: 10.1186/s12964-024-01672-0. Cell Commun Signal. 2024. PMID: 38802896 Free PMC article.
-
Investigation of the effect and mechanism of Fei Re Pu Qing powder in treating acute lung injury (ALI) by modulating macrophage polarization via serum pharmacology and network pharmacology.J Ethnopharmacol. 2025 Jul 24;351:120089. doi: 10.1016/j.jep.2025.120089. Epub 2025 Jun 9. J Ethnopharmacol. 2025. PMID: 40499803
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Zhai Y, Yu T, Xin S, Ding Y, Cui Y, Nie H. Network pharmacology-based research into the mechanism of ferulic acid on acute lung injury through enhancing transepithelial sodium transport. J Ethnopharmacol. 2024;330: 118230. 10.1016/j.jep.2024.118230. - PubMed
-
- Qi X, Luo Y, Xiao M, Zhang Q, Luo J, Ma L, et al. Mechanisms of alveolar type 2 epithelial cell death during acute lung injury. Stem Cells. 2023;41:1113–32. 10.1093/stmcls/sxad074. - PubMed
-
- Bos LDJ, Ware LB. Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes. Lancet. 2022;400:1145–56. 10.1016/S0140-6736(22)01485-4. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

