Reduction in systemic glucocorticoid utilization among COPD patients with type 2 inflammation treated with biologics
- PMID: 40640771
- PMCID: PMC12243432
- DOI: 10.1186/s12890-025-03809-4
Reduction in systemic glucocorticoid utilization among COPD patients with type 2 inflammation treated with biologics
Abstract
Background: Systemic glucocorticoids are associated with significant side effects, however, are essential in the treatment of acute exacerbations of chronic obstructive pulmonary disease (COPD). Biologic therapies in COPD with type 2 (T2) inflammation have shown benefit in reducing exacerbations, but their impact on glucocorticoid utilization remains unclear. We aim to examine if use of biologics in COPD patients reduces glucocorticoid burden.
Methods: A retrospective review of the electronic medical record (2016-2023) was performed. Patients with COPD that were treated with biologics were included. Data collected included demographics, baseline comorbidities, eosinophil count, pulmonary function testing and dispense reports for glucocorticoids. The primary outcomes were a change in the number of glucocorticoid dispenses and total cumulative systemic glucocorticoid dosage, in the year prior and post initiation of therapy.
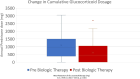
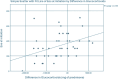
Results: 56 patients (mean age 71 ± 8.5) were included in the study. 55% had coronary artery disease, 25% had heart failure, 71% had hypertension, 14% had stroke and 30% had diabetes. Biologics significantly reduced annual glucocorticoid dispenses (3.38 ± 2.58 vs. 2.22 ± 2.33, mean reduction 1.16, 95% CI 0.45-1.87, p = 0.002) and cumulative dosage (1073 ± 831 mg vs. 659 ± 723 mg, mean reduction 413.2 mg, 95% CI 180.8-645.6, p = 0.001). There was no strong association between baseline eosinophil count and glucocorticoid utilization.
Conclusions: In this real-world cohort of COPD patients with T2 inflammation, the addition of biologic therapies was associated with a significant reduction in systemic glucocorticoid usage, both in terms of dispense frequency and overall total dosage of systemic glucocorticoids. This highlights the potential of biologics to reduce glucocorticoid-related adverse effects in COPD patients.
Keywords: Biologic therapy; COPD; Eosinophilia; Glucocorticoid sparing; Type 2 inflammation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the local ethics committee of Temple University (IRB Protocol #31820). The need for patient consent to participate was waived by the Temple University IRB/ethics committee given the retrospective nature of the study. This study was performed in accordance with the ethical standards of the Helsinki Declaration of 1975 and Western Institutional Review Board. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Sivapalan P, Ingebrigtsen TS, Rasmussen DB, et al. COPD exacerbations: the impact of long versus short courses of oral corticosteroids on mortality and pneumonia: nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Respir Res. 2019;6(1):e000407. 10.1136/bmjresp-2019-000407. - PMC - PubMed
-
- Groenewegen KH, Schols AMWJ, Wouters EFM. Mortality and Mortality-Related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459–67. 10.1378/chest.124.2.459. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical