Recent advances and applications of mitochondria in tumors and inflammation
- PMID: 40640856
- PMCID: PMC12247300
- DOI: 10.1186/s12967-025-06722-w
Recent advances and applications of mitochondria in tumors and inflammation
Abstract
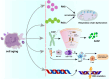
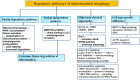
Mitochondria are bilayer membrane organelles with basic metabolic activity. They are considered hubs for biosynthesis, bioenergy, and signaling functions, coordinating major biological pathways. Mitochondria are coupled to the oxidation of fatty acids and pyruvate through electron transport chains and have historically been considered the primary source of cellular energy. Recent studies have depicted that mitochondria are centers that promote inflammatory responses and play a crucial role in combating pathogenic infections. Moreover, mitochondria provide the basis for tumor synthesis metabolism, control redox and calcium homeostasis, participate in transcriptional regulation, and control cell death. Mitochondria are involved in all steps of tumorigenesis. This review discusses the relationship between mitochondria (including mitochondrial metabolism and mitophagy) and tumors, and the relationship between mtDNA and inflammation, as well as its clinical application in inflammatory diseases. More importantly, the application and targeted treatment strategies provide more opportunities for the development of new anticancer drugs.
Keywords: Inflammation; Metabolism; Mitochondria; Tumor.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All authors have approved this manuscript for publication. Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Recent Advances in the Application of Mitochondria-targeted Fluorescent Probes.Curr Med Chem. 2025 Jul 4. doi: 10.2174/0109298673368188250613112621. Online ahead of print. Curr Med Chem. 2025. PMID: 40621768
-
Mitochondrial dysfunction in the regulation of aging and aging-related diseases.Cell Commun Signal. 2025 Jun 19;23(1):290. doi: 10.1186/s12964-025-02308-7. Cell Commun Signal. 2025. PMID: 40537801 Free PMC article. Review.
-
Construction of Z-Scheme MOF-on-MOF heterostructures for mitochondria-targeted sonodynamic therapy.Acta Biomater. 2025 Jun 15;200:653-666. doi: 10.1016/j.actbio.2025.05.001. Epub 2025 May 1. Acta Biomater. 2025. PMID: 40318748
-
Toll-like receptor 4 mutation suppresses hyperhomocysteinemia-induced hypertension.Am J Physiol Cell Physiol. 2016 Oct 1;311(4):C596-C606. doi: 10.1152/ajpcell.00088.2016. Epub 2016 Aug 3. Am J Physiol Cell Physiol. 2016. PMID: 27488663 Free PMC article.
-
Overview of methods that determine mitochondrial function in human disease.Metabolism. 2025 Sep;170:156300. doi: 10.1016/j.metabol.2025.156300. Epub 2025 May 17. Metabolism. 2025. PMID: 40389059 Free PMC article. Review.
References
-
- Oliveira GL, Coelho AR, Marques R, Oliveira PJ. Cancer cell metabolism: rewiring the mitochondrial hub. Biochim Biophys Acta Mol Basis Dis. 2021;1867(2):166016. - PubMed
-
- Liu S, Feng M, Guan W. Mitochondrial DNA sensing by STING signaling participates in inflammation, cancer and beyond. Int J Cancer. 2016;139(4):736–41. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

