Exosomes derived from hypoxia-preconditioned M2 macrophages alleviate degeneration in knee osteoarthritis through the miR‑124‑3p/STAT3 axis
- PMID: 40640893
- PMCID: PMC12247280
- DOI: 10.1186/s12967-025-06808-5
Exosomes derived from hypoxia-preconditioned M2 macrophages alleviate degeneration in knee osteoarthritis through the miR‑124‑3p/STAT3 axis
Abstract
Background: M2 macrophages-derived exosomes (M2φ-Exos) have been demonstrated to effectively alleviate osteoarthritis (OA) in animal models. Hypoxic preconditioning is commonly used to enhance the biological effects of stem cell-derived exosomes, but its impact on M2φ-Exos remains unclear. This study aims to investigate whether hypoxic preconditioning could enhance the biological effects of M2φ-Exos in OA treatment and to explore the underlying molecular mechanisms, with the goal of providing new insights for the development of safe and effective OA therapeutic strategies.
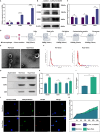
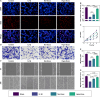
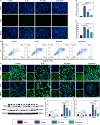
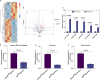
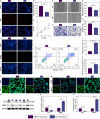
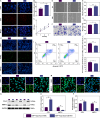
Methods: Exosomes were extracted from the supernatants of M2 macrophages cultured under normoxic or hypoxic conditions using low-temperature differential ultracentrifugation and were designated as Nor-Exos and Hypo-Exos. The exosomes were characterized by transmission electron microscopy, nanoparticle tracking analysis, and Western blotting. To evaluate the impact of hypoxic preconditioning on the biological effects of M2φ-Exos, the therapeutic effects of Nor-Exos and Hypo-Exos were assessed in an IL-1β-induced chondrocyte inflammation model and a rat knee OA model established by surgical intervention. Exosomal miRNAs with differential expression between Nor-Exos and Hypo-Exos were identified through exosomal miRNA sequencing. The miRNA with the highest upregulation in Hypo-Exos was selected for further functional validation. To investigate the role of this miRNA, miRNA inhibitors were used to knock down its expression in Hypo-Exos, and the subsequent impact of this change on Hypo-Exos activity was evaluated. Bioinformatic tools and dual-luciferase reporter assays were used to predict and verify the downstream target genes of the miRNA. Target gene expression was knocked down using small interfering RNA, and the effect of downregulating target gene expression on the inhibitory effect of low miRNA expression on Hypo-Exos was observed at the cellular level.
Results: Compared to Nor-Exos, Hypo-Exos exhibited more effective therapeutic effects in both inflammatory chondrocytes and OA rats. miR-124-3p was identified as the most upregulated miRNA in Hypo-Exos, and the suppression of miR-124-3p expression significantly inhibited the biological effects of Hypo-Exos. STAT3 was determined to be a downstream target gene of miR-124-3p. Further cellular experiments revealed that downregulation of STAT3 expression in chondrocytes successfully alleviated the inhibitory effect of low miR-124-3p expression on the biological effects of Hypo-Exos.
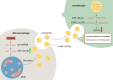
Conclusions: Hypoxic preconditioning enhances the biological effects of M2φ-Exos in the treatment of OA. The underlying molecular mechanism is associated with increased delivery of miR-124-3p to chondrocytes, which subsequently inhibits the post-transcriptional expression of STAT3. This provides a promising therapeutic strategy for the clinical intervention of OA.
Keywords: Exosomes; Hypoxic preconditioning; M2 macrophages; Osteoarthritis; STAT3; miR-124-3p.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experimental procedures involving in animals was approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (DW2024002). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



Similar articles
-
Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126.Acta Biomater. 2020 Feb;103:196-212. doi: 10.1016/j.actbio.2019.12.020. Epub 2019 Dec 17. Acta Biomater. 2020. PMID: 31857259
-
Exosomes-Shuttled lncRNA SNHG7 by Bone Marrow Mesenchymal Stem Cells Alleviates Osteoarthritis Through Targeting miR-485-5p/FSP1 Axis-Mediated Chondrocytes Ferroptosis and Inflammation.Tissue Eng Regen Med. 2024 Dec;21(8):1203-1216. doi: 10.1007/s13770-024-00668-8. Epub 2024 Oct 3. Tissue Eng Regen Med. 2024. PMID: 39363054
-
Increased levels of villus-derived exosomal miR-29a-3p in normal pregnancy than uRPL patients suppresses decidual NK cell production of interferon-γ and exerts a therapeutic effect in abortion-prone mice.Cell Commun Signal. 2024 Apr 16;22(1):230. doi: 10.1186/s12964-024-01610-0. Cell Commun Signal. 2024. PMID: 38627796 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
WITHDRAWN: Non-aspirin, non-steroidal anti-inflammatory drugs for treating osteoarthritis of the knee.Cochrane Database Syst Rev. 2007 Jul 18;2006(1):CD000142. doi: 10.1002/14651858.CD000142.pub2. Cochrane Database Syst Rev. 2007. PMID: 17636601 Free PMC article.
References
-
- Gelber AC. Knee osteoarthritis. Ann Intern Med. 2024;177(9):Itc129–44. - PubMed
-
- Courties A, Kouki I, Soliman N, Mathieu S, Sellam J. Osteoarthritis year in review 2024: epidemiology and therapy. Osteoarthritis Cartilage. 2024;32(11):1397–404. - PubMed
-
- Tang S, Zhang C, Oo WM, Fu K, Risberg MA, Bierma-Zeinstra SM, et al. Osteoarthr Nat Rev Dis Primers. 2025;11(1):10. - PubMed
-
- Jenei-Lanzl Z, Zaucke F. Osteoarthritis year in review 2024: biology. Osteoarthritis Cartilage. 2025;33(1):58–66. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

