The solute carrier family 11 transporters: a bridge between iron homeostasis and tumor biology
- PMID: 40640903
- PMCID: PMC12247208
- DOI: 10.1186/s12964-025-02293-x
The solute carrier family 11 transporters: a bridge between iron homeostasis and tumor biology
Abstract
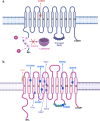
Iron is an essential trace element in the human body, and its imbalance is closely linked to the initiation and progression of various malignancies. The solute carrier family 11 (SLC11) transporters, comprising SLC11A1 and SLC11A2, play pivotal roles in iron metabolism and cellular homeostasis, processes intricately linked to oncogenesis. SLC11A1, primarily expressed in macrophages, modulates immune responses and reshapes the tumor microenvironment, while SLC11A2, a ubiquitous iron transporter, regulates dietary iron absorption and ferroptosis, an iron-dependent form of programmed cell death. Dysregulation of these transporters is associated with tumor initiation, progression, metastasis, and therapy resistance. In this review, we provide an overview of the physiological functions of SLC11 transporters in iron metabolism and their pathological roles in cancer biology. Emerging evidence highlights their involvement in key oncogenic pathways, including p53, JAK/STAT, Wnt and HIF signaling. Pharmacological and genetic interventions targeting SLC11 transporters have shown the potential to disrupt tumor progression and enhance treatment efficacy. By exploring the intricate roles of SLC11A1 and SLC11A2 in cancer progression, this review offers insights into their potential as biomarkers and therapeutic targets, paving the way for innovative cancer treatment strategies.
Keywords: Cancer; Iron; Iron transporters; SLC11A1; SLC11A2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Protein iron transporters as potential therapeutic targets in cancer: A review.Int J Biol Macromol. 2025 Aug 7:146695. doi: 10.1016/j.ijbiomac.2025.146695. Online ahead of print. Int J Biol Macromol. 2025. PMID: 40782860 Review.
-
Hepcidin: A multifaceted hormone in iron homeostasis and tumor biology.Vitam Horm. 2025;129:317-360. doi: 10.1016/bs.vh.2024.10.003. Epub 2024 Oct 24. Vitam Horm. 2025. PMID: 40812950 Review.
-
An Analysis of AMPK and Ferroptosis in Cancer: A Potential Regulatory Axis.Front Biosci (Landmark Ed). 2025 Jul 30;30(7):36618. doi: 10.31083/FBL36618. Front Biosci (Landmark Ed). 2025. PMID: 40765336 Review.
-
Targeting Ferroptosis With Natural Products to Treat Diabetes and Its Complications: Opportunities and Challenges.Phytother Res. 2025 Jul 9. doi: 10.1002/ptr.70028. Online ahead of print. Phytother Res. 2025. PMID: 40634143 Review.
-
EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update.Eur J Cancer. 2007 Jan;43(2):258-70. doi: 10.1016/j.ejca.2006.10.014. Epub 2006 Dec 19. Eur J Cancer. 2007. PMID: 17182241
References
-
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–97. - PubMed
-
- Prousek J. Fenton chemistry in biology and medicine. Pure Appl Chem. 2007;79:2325–38.
-
- Jomova K, Valko M. Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des. 2011;17:3460–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

