USP5-Mediated PD-L1 deubiquitination regulates immunotherapy efficacy in melanoma
- PMID: 40640907
- PMCID: PMC12247207
- DOI: 10.1186/s12967-025-06812-9
USP5-Mediated PD-L1 deubiquitination regulates immunotherapy efficacy in melanoma
Abstract
Background: The role of post-translational modifications(PTMs) in PD-L1-mediated immune resistance and melanoma progression remains poorly understood.
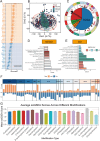
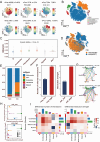
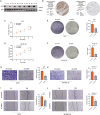
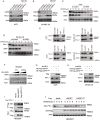
Methods: We conducted multi-omics analyses and constructed a prognostic model based on PTM-related genes using machine learning to identify key regulators in melanoma. In vitro and in vivo experiments, including cell culture, flow cytometry, and subcutaneous allografts models, were used to investigate USP5's function. Protein-protein interactions were validated using Western blotting and co-immunoprecipitation, while PD-L1 stability and ubiquitination were assessed using cycloheximide (CHX) chase and ubiquitination assays.
Results: USP5 was identified as a key DUB that specifically deubiquitinates K48-linked polyubiquitin chains on PD-L1, stabilizing its protein levels. USP5 knockdown reduced PD-L1 expression, enhanced CD8 + T-cell infiltration and activation, and suppressed melanoma progression in both in vitro and in vivo models. Furthermore, combining USP5 knockdown with anti-PD-1 therapy significantly improved therapeutic efficacy by reducing tumor burden and promoting T-cell activation.
Conclusion: USP5 promotes immune escape in melanoma by stabilizing PD-L1 through deubiquitination, representing a novel mechanism hindering the efficacy of ICIs. Targeting USP5 could enhance anti-PD-1 therapy and improve patient outcomes. These findings underscore the therapeutic potential of USP5 inhibition as a strategy to overcome immune resistance in melanoma.
Keywords: Immune escape; Immunotherapy; Melanoma; PD-L1; USP5.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The collection of specimens and animal handling for the study have been reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University(2406070 and 2024-SR-949) and Ethics Committee of Xiangya Hospital of Central South University(2024030015). Competing interests: The authors declare no competing interests.
Figures



References
-
- Tagore M, Hergenreder E, Perlee SC, et al. GABA regulates electrical activity and tumor initiation in melanoma. Cancer Discov. 2023;13(10):2270–91. 10.1158/2159-8290.CD-23-0389 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

