Reevaluating antiviral thresholds in HBV DNA-negative inactive HBsAg carriers: a multicenter histopathological analysis
- PMID: 40640910
- PMCID: PMC12243403
- DOI: 10.1186/s12985-025-02853-0
Reevaluating antiviral thresholds in HBV DNA-negative inactive HBsAg carriers: a multicenter histopathological analysis
Abstract
Background: The definition of inactive HBsAg carriers (IHC) varies globally, particularly regarding HBV DNA thresholds. Whether HBV DNA negativity reliably predicts histological quiescence remains uncertain.
Aims: This study evaluated liver pathology in IHC patients to reassess antiviral therapy thresholds.
Methods: This multi-center, retrospective study included 231IHCs(2018-2023) stratified by HBV DNA negativity (< 20IU/mL). Liver biopsies assessed inflammation (G ≥ 2) and fibrosis (F ≥ 2); evident hepatic injury (EHI) was defined as G ≥ 2 and/or F ≥ 2. Multivariable models evaluated predictors.
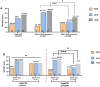
Results: Among 231 IHC patients(median age:43 years old; 95.2% ≥30 years old), 35.9%(83/231) were HBV DNA negative. The median HBsAg and HBV DNA level were 132 IU/ml and 94 IU/ml, respectively. Notably, EHI prevalence was significantly higher in HBV DNA negative patients than positive ones(44.9% vs. 31%, P = 0.04), driven by fibrosis (F ≥ 2: 42.2% vs. 21.6%, P < 0.001), challenging the assumption that HBV DNA negativity ensures low histological risk. Male sex, HBV DNA negativity, and elevated liver stiffness measurement(LSM) independently predicted EHI (OR = 3.37, AUC = 0.747).
Conclusion: HBV DNA negativity does not guarantee histological quiescence in inactive HBsAg carriers aged ≥ 30 years, with 44.9% exhibiting significant liver injury. In this population, LSM > 6.4 Kpa should prompt consideration of liver biopsy and/or initiation of antiviral therapy.
Keywords: Antiviral thresholds; Chronic hepatitis B; Fibrosis; Inactive HBsAg carriers; Liver histopathology; Non-invasive biomarkers.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by the Ethics Committee of Beijing Youan Hospital, Capital Medical University (Approval Code: [2022]159), in accordance with the 1975 Helsinki Declaration (revised 1983). Written informed consent was obtained from all participants. Consent for publication: Not applicable (retrospective analysis of anonymized data). Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
[Analysis of liver histological characteristics and clinically related factors in patients with inactive HBsAg carriers].Zhonghua Gan Zang Bing Za Zhi. 2025 Jul 20;33(7):660-666. doi: 10.3760/cma.j.cn501113-20241115-00580. Zhonghua Gan Zang Bing Za Zhi. 2025. PMID: 40784802 Chinese.
-
Clinical outcomes of untreated adults living with chronic hepatitis B in The Gambia: an analysis of data from the prospective PROLIFICA cohort study.Lancet Gastroenterol Hepatol. 2024 Dec;9(12):1133-1146. doi: 10.1016/S2468-1253(24)00226-7. Lancet Gastroenterol Hepatol. 2024. PMID: 39521002
-
VIR-2218 (elebsiran) plus pegylated interferon-alfa-2a in participants with chronic hepatitis B virus infection: a phase 2 study.Lancet Gastroenterol Hepatol. 2024 Dec;9(12):1121-1132. doi: 10.1016/S2468-1253(24)00237-1. Epub 2024 Oct 8. Lancet Gastroenterol Hepatol. 2024. PMID: 39389081 Clinical Trial.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.Health Technol Assess. 2006 Aug;10(28):iii-iv, xi-xiv, 1-183. doi: 10.3310/hta10280. Health Technol Assess. 2006. PMID: 16904047
-
Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus.Cochrane Database Syst Rev. 2017 Feb 11;2(2):CD008545. doi: 10.1002/14651858.CD008545.pub2. Cochrane Database Syst Rev. 2017. PMID: 28188612 Free PMC article.
References
-
- European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98. 10.1016/j.jhep.2017.03.021 - PubMed
-
- Chinese Society of Hepatology, Chinese Society of Infectious Diseases. Guidelines for the prevention and treatment of chronic hepatitis B (2022 version). Chin J Clin Infect Dis. 2022;15(6):401–27. 10.3760/cma.j.issn.1674-2397.2022.06.001
Publication types
MeSH terms
Substances
Grants and funding
- 2024-2-2184/Capital's Funds for Health Improvement and Research
- 2023YFC2308100/National Key Research and Development Program of Ministry of Science and Technology
- Academic Leader -02-14/High-level public health technical talents construction project of Beijing Municipal Health Commission
- Z211100002921059/Capital Clinical Diagnostic Techniques and Translational Application Projects
LinkOut - more resources
Full Text Sources

