Restoring brain barriers: an innovative approach for treating neurological disorders
- PMID: 40640916
- PMCID: PMC12243347
- DOI: 10.1186/s12987-025-00688-z
Restoring brain barriers: an innovative approach for treating neurological disorders
Abstract
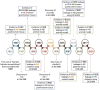
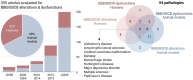
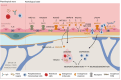
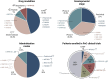
The complex etiology of neurological disorders is a major challenge to the identification of therapeutic candidates. Tackling brain vascular dysfunction is gaining attention from the scientific community, neurologists and pharmaceutical companies, as a novel disease-modifying strategy. Here, we provide evidence that at least 41% of neurological diseases and related conditions/injuries display a co-pathology of blood-brain and blood-spinal cord barrier alterations and dysfunctions, and we discuss why this figure may represent only a fraction of a larger phenomenon. We further provide clinical evidence that barrier status may contribute to pathological and functional outcomes in patients. Finally, we discuss drug candidates under development to repair brain barriers.
Keywords: Alzheimer’s disease; Amyotrophic lateral sclerosis; BBB disruption; BSCB disruption; Claudin-5; Huntington’s disease; Multiple sclerosis; Parkinson’s disease; Therapeutic strategies; Tight junction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: SL and AJ are employed by Axoltis Pharma and are respectively chief scientific officer and chief medical officer of the company. YG is chief executive officer and a shareholder of Axoltis Pharma. BE, GB, FC and MC are members of the scientific advisory board (SAB) of Axoltis Pharma. BE and MC are members of the SAB of Neuvasq Biotechnologies. As employees, chief executive officer, or SAB members of companies working on drug candidates for barrier repair in neurological disorders, we have deliberately chosen to stay neutral on the third part of this review by just reporting factual elements without interpretation and comparison of the mechanisms of action of the drug candidates being developed, to avoid any conflict of interest. Ethics approval: Not applicable. Consent to publication: Not applicable. Consent to participate: Not applicable.
Figures


References
-
- Faden AI, Stoica B. Neuroprotection: challenges and opportunities. Arch Neurol. 2007;64(6):794–800. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

