Gut microbiota-derived indoleacetic acid attenuates neuroinflammation and neurodegeneration in glaucoma through ahr/rage pathway
- PMID: 40640940
- PMCID: PMC12243265
- DOI: 10.1186/s12974-025-03505-4
Gut microbiota-derived indoleacetic acid attenuates neuroinflammation and neurodegeneration in glaucoma through ahr/rage pathway
Abstract
Background: Gut microbiota has emerged as a promising therapeutic target for neurodegenerative disorders through regulation of neuroinflammatory responses, while its role in optic nerve degeneration remains incompletely characterized. This study elucidates the neuroprotective role of gut microbiota derived tryptophan metabolites in glaucoma through gut-eye communication and inhibition of microglia-mediated neuroinflammation.
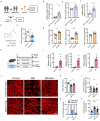
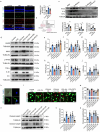
Methods: Gut microbiota profiling (16 S rRNA sequencing) and serum indoleacetic acid (IAA) quantification were performed in glaucoma patients versus controls. Microbiota-metabolite relationships were further validated through fecal microbiota transplantation (FMT). The neuroprotective and anti-neuroinflammatory effect of Bacteroides fragilis (B. fragilis) and IAA was assessed in both microbead-induced ocular hypertension mice model and in vitro BV-2 microglial cell inflammation model via immunofluorescence, qPCR, Western blot and mice behavioral assays. To explore the underlying mechanisms, retinal transcriptomics and microglia-neuron co-cultures were also employed.
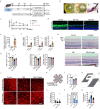
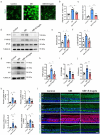
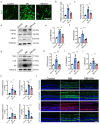
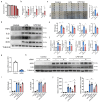
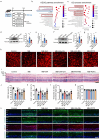
Result: Glaucoma patients exhibited gut dysbiosis characterized by depleted tryptophan-metabolizing bacteria (B. fragilis, Bacteroides thetaiotaomicron, Anaerostipes hadrus) and reduced serum IAA levels. Mice receiving FMT from glaucoma patients exhibited lower systemic IAA levels. In in vivo and in vitro models, B. fragilis or IAA restored AhR activation, suppressed inflammation by inhibiting microglial activation and the release of pro-inflammatory mediators throughout the retina, reduced retinal ganglion cells (RGCs) loss and preserved visual function. Mechanistically, IAA attenuated RAGE/NF-κB pathway activation via AhR-dependent signaling, conferring neuroprotection.
Conclusion: Our study proposes a novel AhR-mediated gut microbiota-eye axis in glaucoma pathogenesis and demonstrates that IAA serves as an effective neuroprotective strategy with clinical potential for managing RGCs neurodegeneration.
Keywords: AhR; Glaucoma; Gut microbiota-eye axis; Microglia; Neuroinflammation; Tryptophan metabolites.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Committee of Animal Care of the Shanghai 9th People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine (Approval no. SH9H-2022-A16-2). All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology’s Statement for Use of Animals. The protocol for human serum collection were approved by the institutional review board of Ninth People’s hospital affiliated with the Shanghai Jiao Tong University School of Medicine (No. SH9H-2021-T184-2) and informed consent was obtained from all subjects. Consent for publication: All consent included in this study are available upon request by contact with the corresponding author. Competing interests: The authors declare no competing interests.
Figures


References
-
- Hassan A, Huang Y, Mahjabina, Sudiro OH, Wang X, He J, Wei Y, Liao X, Wang G. Emerging role of gut microbiota extracellular vesicle in neurodegenerative disorders and insights on their therapeutic management. iMetaOmics 1. 10.1002/imo2.33 (2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

