African swine fever virus infection of porcine peripheral blood monocyte-derived macrophages induces the formation of tunneling nanotube-connected large vesicle-like cell segments: a potential mechanism for intercellular ASFV trafficking
- PMID: 40640946
- PMCID: PMC12247315
- DOI: 10.1186/s13567-025-01582-0
African swine fever virus infection of porcine peripheral blood monocyte-derived macrophages induces the formation of tunneling nanotube-connected large vesicle-like cell segments: a potential mechanism for intercellular ASFV trafficking
Abstract
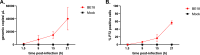
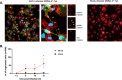
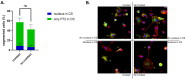
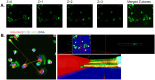
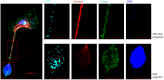
African swine fever (ASF) is a highly fatal viral disease in pigs, with mortality rates that can reach 100%. The causative agent, African swine fever virus (ASFV), primarily targets cells of the mononuclear phagocytic system (MPS), particularly monocyte-derived macrophages (MDMs). Despite the severity of the disease, there are currently no effective antiviral treatments available in Europe. A significant barrier to therapeutic development is the limited understanding of how ASFV interacts with its primary target cells. A deeper understanding of the morphological changes induced by ASFV in infected cells is crucial to this effort. To address this knowledge gap, we used conventional and confocal immunofluorescence microscopy, as well as transmission electron microscopy, to investigate ASFV-infected primary MDMs. Our analysis revealed that ASFV infection leads to the formation of large cellular protrusions, which are characterized by vesicle-shaped cellular segments (CSs) at their tips. These protrusions contain all major cytoskeletal components, showing characteristics similar to those of tunneling nanotubes (TNTs). In 84.93% of the cases, the nucleus remained in the cell body (CB) near the viral factory. In the remaining cases, the nucleus was found within these CSs, whereas the viral factory was present in the CB. Additionally, 57.6% of the cells were in contact with the CS and distant cells, suggesting a potential mechanism for ASFV transmission. These findings suggest that ASFV induces cellular segmentation linked by TNT-like structures. Further research is needed to better understand the biogenesis and functional significance of these segmented cells, which could inform future strategies for combating ASFV.
Keywords: African swine fever virus; cytopathic effect; tunneling nanotubes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The activities in the BSL-3 laboratory were authorized by Brussels Environment under reference number SBB 219 2020/0493. Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
African swine fever virus infection enhances CD14-dependent phagocytosis of porcine alveolar macrophages to promote bacterial uptake and apoptotic body-mediated viral transmission.J Virol. 2025 Jul 22;99(7):e0069025. doi: 10.1128/jvi.00690-25. Epub 2025 Jun 12. J Virol. 2025. PMID: 40503879 Free PMC article.
-
From hemorrhage to apoptosis: understanding the devastating impact of ASFV on piglets.Microbiol Spectr. 2025 Aug 5;13(8):e0290224. doi: 10.1128/spectrum.02902-24. Epub 2025 Jul 11. Microbiol Spectr. 2025. PMID: 40642987 Free PMC article.
-
Revisiting the early event of African swine fever virus DNA replication.J Virol. 2025 Jul 22;99(7):e0058425. doi: 10.1128/jvi.00584-25. Epub 2025 May 30. J Virol. 2025. PMID: 40444991 Free PMC article.
-
Risk factors for the spread of African Swine Fever in China: A systematic review of Chinese-language literature.Transbound Emerg Dis. 2022 Sep;69(5):e1289-e1298. doi: 10.1111/tbed.14573. Epub 2022 May 11. Transbound Emerg Dis. 2022. PMID: 35490407 Free PMC article.
-
African Swine Fever Virus Circulation between Tanzania and Neighboring Countries: A Systematic Review and Meta-Analysis.Viruses. 2021 Feb 15;13(2):306. doi: 10.3390/v13020306. Viruses. 2021. PMID: 33672090 Free PMC article.
References
-
- Eustace Montgomery R (1921) On a form of swine fever occurring in British East Africa (Kenya Colony). J Comp Pathol Ther 34:159–191
-
- Venkateswaran D, Prakash A, Nguyen QA, Salman M, Suntisukwattana R, Atthaapa W, Tantituvanont A, Lin H, Songkasupa T, Nilubol D (2024) Comprehensive characterization of the genetic landscape of African swine fever virus: insights into infection dynamics, immunomodulation, virulence and genes with unknown function. Animals 14:2187 - PMC - PubMed
-
- Dixon LK, Chapman DAG, Netherton CL, Upton C (2013) African swine fever virus replication and genomics. Virus Res 173:3–14 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

