LncRNA SDCBP2-AS1 is a putative biomarker for postmenopausal osteoporosis and promotes osteogenic differentiation of BMSCs by regulating miR-361-3p
- PMID: 40640983
- PMCID: PMC12247319
- DOI: 10.1186/s41065-025-00494-5
LncRNA SDCBP2-AS1 is a putative biomarker for postmenopausal osteoporosis and promotes osteogenic differentiation of BMSCs by regulating miR-361-3p
Abstract
Background: Numerous long noncoding RNAs (lncRNAs) have been proven to participate in osteogenesis and postmenopausal osteoporosis (PMOP). We measured serum SDCBP2-AS1 expression changes in patients with PMOP and investigated its effects on osteoblast differentiation in human bone marrow-derived mesenchymal stem cells (hBMSC) cells.
Methods: RT-qPCR was used to measure SDCBP2-AS1 levels and the expression of osteogenic differentiation indicators. The diagnostic efficacy of SDCBP2-AS1 was assessed using a receiver operating characteristic (ROC) analysis. CCK-8 and flow cytometry methods were employed to investigate the functional impact of SDCBP2-AS1 on hBMSC cell proliferation and apoptosis during osteoblast differentiation. The bioinformatics, dual-luciferase reporter assay, and RNA Immunoprecipitation (RIP) assay were used to identify and confirm SDCBP2-AS1/miR-361-3p interaction.
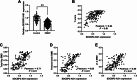
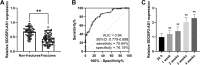
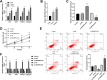
Results: Serum SDCBP2-AS1 was decreased in patients with PMOP, especially in those with fractures. The SDCBP2-AS1 levels were positively correlated with patients' T scores and BMDs. Decreased SDCBP2-AS1 had a certain high area under the ROC curve (AUC) value (AUC = 0.81) in distinguishing PMOP patients with fractures from those without fractures. SDCBP2-AS1 levels gradually increased after four weeks of treatment in PMOP patients and hBMSCs during cell differentiation. Enhanced SDCBP2-AS1 promoted cell proliferation and the levels of osteoblast differentiation markers, including ALP, OCN, RUNX2, and Collagen I, while decreasing cell apoptosis. miR-361-3p was a direct target of SDCBP2-AS1. The influence of SDCBP2-AS1 on cell activities and hBMSCs differentiation was diminished by miR-361-3p.
Conclusions: SDCBP2-AS1 might be a diagnostic biomarker in predicting PMOP patients with fractures. By measuring the levels of SDCBP2-AS1 in patient samples, clinicians may be able to identify those who are more susceptible to bone fractures, enabling earlier and more targeted preventive measures. SDCBP2-AS1 targeting miR-361-3p regulates the osteogenic differentiation of hBMSCs, which might be a new target for the treatment of PMOP.
Keywords: BMSC; Diagnosis; Osteoporosis; Postmenopausal; SDCBP2-AS1; miR-361-3p.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The procedures used in this study adhere to the tenets of the Declaration of Helsinki. All individuals involved in the study were advised of its objectives and provided signed consent forms. The research protocol received ethical clearance from the Ethics Committee at The First Affiliated Hospital of Xiamen University. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Subarajan P, Arceo-Mendoza RM, Camacho PM. Postmenopausal osteoporosis: A review of latest guidelines. Endocrinol Metab Clin North Am. 2024;53(4):497–512. - PubMed
-
- Gao S, Xu T, Wang W, Li J, Shan Y, Wang Y, et al. Polysaccharides from anemarrhena asphodeloides bge, the extraction, purification, structure characterization, biological activities and application of a traditional herbal medicine. Int J Biol Macromol. 2025;311(Pt 1):143497. - PubMed
-
- Arthur Vithran DT, Essien AE, Rahmati M, Opoku M, Keon Yon D, López Sánchez GF, et al. Teriparatide in postmenopausal osteoporosis: Uncovering novel insights into efficacy and safety compared to other treatments - a systematic review and meta-analysis. EFORT Open Reviews. 2024;9(9):845–61. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

