Synthesis of gem-Difluorocyclobutanes: Organolanthanum Enabled Synthesis and Divergent Catalytic Functionalization of gem-Difluorocyclobutanols
- PMID: 40640992
- PMCID: PMC12305666
- DOI: 10.1021/acs.joc.5c01175
Synthesis of gem-Difluorocyclobutanes: Organolanthanum Enabled Synthesis and Divergent Catalytic Functionalization of gem-Difluorocyclobutanols
Abstract
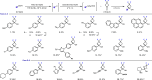
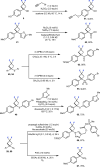
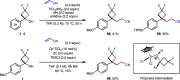
Fluorinated cycloalkyl motifs continue to receive intense interest in medicinal chemistry due to their potential to modulate physicochemical properties. In recent years, this interest has extended to gem-difluorocyclobutanes as small, polar, yet lipophilic moieties. However, strategies to access gem-difluorocyclobutanes remain limited, presenting opportunities for the development of new synthetic methods. Here, we report the synthesis and divergent functionalization of gem-difluorocyclobutanols to generate a diverse range of 1,1-disubstituted-3,3-difluorocyclobutanes. The use of organolanthanum reagents is crucial to achieve the addition of carbon nucleophiles to commercially available difluorocyclobutanone to avoid the undesired elimination of HF by controlling nucleophile basicity. The generated difluorocyclobutanols enable functionalization through carbocation and radical intermediates, providing diverse 1,1-disubstituted difluorocyclobutanes and expediting access to new design options for medicinal or materials chemistry.
Figures


References
-
- Berger R., Resnati G., Metrangolo P., Weber E., Hulliger J.. Organic Fluorine Compounds: A Great Opportunity for Enhanced Materials Properties. Chem. Soc. Rev. 2011;40(7):3496–3508. doi: 10.1039/c0cs00221f. - DOI - PubMed
- Ogawa Y., Tokunaga E., Kobayashi O., Hirai K., Shibata N.. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience. 2020;23(9):101467. doi: 10.1016/j.isci.2020.101467. - DOI - PMC - PubMed
- Du Y., Bian Y., Baecker D., Dhawan G., Semghouli A., Kiss L., Zhang W., Sorochinsky A. E., Soloshonok V. A., Han J.. Fluorine in the Pharmaceutical Industry: FDA-Approved Fluorine-Containing Drugs in 2024. Chem.Eur. J. 2025;31(25):e202500662. doi: 10.1002/chem.202500662. - DOI - PubMed
-
- Gillis E. P., Eastman K. J., Hill M. D., Donnelly D. J., Meanwell N. A.. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015;58(21):8315–8359. doi: 10.1021/acs.jmedchem.5b00258. - DOI - PubMed
- Meanwell N. A.. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018;61(14):5822–5880. doi: 10.1021/acs.jmedchem.7b01788. - DOI - PubMed
- Jeffries B., Wang Z., Felstead H. R., Le Questel J.-Y., Scott J. S., Chiarparin E., Graton J., Linclau B.. Systematic Investigation of Lipophilicity Modulation by Aliphatic Fluorination Motifs. J. Med. Chem. 2020;63(3):1002–1031. doi: 10.1021/acs.jmedchem.9b01172. - DOI - PubMed
- Lesch B., Birkmann A., Bonsmann S., Donald A., Engel F., Goldner T., Kumar K., Pfaff T., Urban A., Zimmermann H., Bosnidou A. E., Del Castillo E., Cuevas F., Detta E., Font D., García J. G., Raymond J., Sarmentero M. A. ´., Cnossen A., Sinnige W., Slootweg J. C., Wegert A., Buschmann H.. Discovery of AIC263282, a Hepatitis B Virus Capsid Assembly Modulator Ready for Candidate Profiling. J. Med. Chem. 2025;68(6):6361–6371. doi: 10.1021/acs.jmedchem.4c02838. For very recent examples in medicinal chemistry programmes, see. - DOI - PubMed
- Miao L., Lou J., Xu S., Zhang J., Zhong Y., Wang G., Li Y., Lei S., Shao S., Wang J., Huang Y., Tang X., Ding W., Ma Z.. Discovery of New Difluorocyclobutyl Derivatives as Effective Glucagon-Like Peptide-1 Receptor Agonists with Reduced hERG Inhibitory Activities. J. Med. Chem. 2025;68(7):7662–7692. doi: 10.1021/acs.jmedchem.5c00231. - DOI - PubMed
-
- Dalvit C., Invernizzi C., Vulpetti A.. Fluorine as a Hydrogen-Bond Acceptor: Experimental Evidence and Computational Calculations. Chem.Eur. J. 2014;20(35):11058–11068. doi: 10.1002/chem.201402858. - DOI - PubMed
- Pollock J., Borkin D., Lund G., Purohit T., Dyguda-Kazimierowicz E., Grembecka J., Cierpicki T.. Rational Design of Orthogonal Multipolar Interactions with Fluorine in Protein–Ligand Complexes. J. Med. Chem. 2015;58(18):7465–7474. doi: 10.1021/acs.jmedchem.5b00975. - DOI - PMC - PubMed
- Zafrani Y., Yeffet D., Sod-Moriah G., Berliner A., Amir D., Marciano D., Gershonov E., Saphier S.. Difluoromethyl Bioisostere: Examining the “Lipophilic Hydrogen Bond Donor” Concept. J. Med. Chem. 2017;60(2):797–804. doi: 10.1021/acs.jmedchem.6b01691. - DOI - PubMed
- Xing L., Keefer C., Brown M. F.. Fluorine Multipolar Interaction: Toward Elucidating Its Energetics in Binding Recognition. J. Fluorine Chem. 2017;198:47–53. doi: 10.1016/j.jfluchem.2016.12.013. - DOI
-
- Hui C., Liu Y., Jiang M., Wu P.. Cyclobutane-Containing Scaffolds in Bioactive Small Molecules. Trends Chem. 2022;4(8):677–681. doi: 10.1016/j.trechm.2022.04.006. - DOI
- Li J., Gao K., Bian M., Ding H.. Recent Advances in the Total Synthesis of Cyclobutane-Containing Natural Products. Org. Chem. Front. 2020;7(1):136–154. doi: 10.1039/C9QO01178A. - DOI
- Hui C., Wang Z., Xie Y., Liu J.. Contemporary Synthesis of Bioactive Cyclobutane Natural Products. Green Synth. Catal. 2023;4(1):1–6. doi: 10.1016/j.gresc.2022.04.006. - DOI
-
- Bauer M. R., Di Fruscia P., Lucas S. C. C., Michaelides I. N., Nelson J. E., Storer R. I., Whitehurst B. C.. Put a Ring on It: Application of Small Aliphatic Rings in Medicinal Chemistry. RSC Med. Chem. 2021;12(4):448–471. doi: 10.1039/D0MD00370K. - DOI - PMC - PubMed
- Van Der Kolk M. R., Janssen M. A. C. H., Rutjes F. P. J. T., Blanco-Ania D.. Cyclobutanes in Small-Molecule Drug Candidates. ChemMedChem. 2022;17(9):e202200020. doi: 10.1002/cmdc.202200020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

