Chimeric PD‑1 receptor redirects primary T cells against childhood solid tumors but not to PD‑1 ligand‑positive CD80‑coexpressing cells
- PMID: 40641108
- PMCID: PMC12272151
- DOI: 10.3892/mmr.2025.13608
Chimeric PD‑1 receptor redirects primary T cells against childhood solid tumors but not to PD‑1 ligand‑positive CD80‑coexpressing cells
Abstract
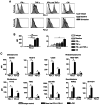
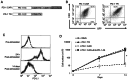
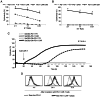
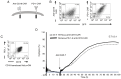
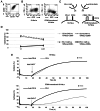
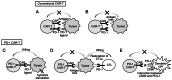
The clinical application of T cells engineered with chimeric antigen receptors (CARs) for solid tumors is challenging. A major reason for this involves tumor immune evasion mechanisms, including the high expression of immune checkpoint molecules, such as the programmed death 1 (PD‑1) ligands PD‑L1 and PD‑L2. The inducible expression of PD‑L1 in tumors has been observed after CAR‑T‑cell infusion, even in tumors natively not expressing PD‑L1. Furthermore, numerous types of pediatric cancer do not have suitable targets for CAR‑T‑cell therapy. Therefore, the present study aimed to develop novel CAR‑T cells that target PD‑L1 and PD‑L2, and to evaluate their efficacy against pediatric solid tumors. A novel CAR harboring the immunoglobulin V‑set domain of the human PD‑1 receptor as an antigen binding site (PD‑1 CAR‑T) was developed without using a single‑chain variable fragment. PD‑1 CAR‑T cells were successfully manufactured by adding an anti‑PD‑1 antibody, nivolumab, to the ex vivo expansion culture to prevent fratricide during the manufacturing process due to the inducible expression of PD‑L1 in activated human T cells. The expression of PD‑L1 (and PD‑L2 to a lesser extent) was revealed to be highly upregulated in various pediatric solid tumor cells, which displayed no or very low expression initially, on in vitro exposure to interferon‑γ and/or tumor necrosis factor‑α, which are cytokines secreted by tumor‑infiltrating T cells. Furthermore, PD‑1 CAR-T cells exhibited strong cytotoxic activity against pediatric solid tumor cells expressing PD‑L1 and PD‑L2. Conversely, the effect of PD‑1 CAR‑T cells was significantly attenuated against PD‑L1‑positive cells coexpressing CD80, suggesting that the toxicity of PD‑1 CAR‑T cells to normal immune cells, including antigen presenting cells, can be minimized. In conclusion, PD‑1 ligands are promising therapeutic targets for pediatric solid tumors. PD‑1 CAR‑T cells, either alone or in combination with CAR‑T cells with other targets, represent a potential treatment option for solid tumors.
Keywords: CD80; PD‑1; PD‑1 ligands; childhood solid tumors; fratricide.
Conflict of interest statement
CI reports patent royalties (patent title: Chimeric receptors with 4-1BB stimulatory signaling domain) from Juno Therapeutics and research funds and advisory fees from CURED, Inc. YS was an employee of CURED, Inc. from February 2022 to January 2024. The other authors declare that they have no competing interests.
Figures
References
-
- Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai YT, Prabhala R, Alonso A, Sperling AS, Campbell T, Petrocca F, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun. 2021;12:868. doi: 10.1038/s41467-021-21177-5. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

