Widespread activation and critical role of EMT and stemness in the neuroendocrine differentiation of prostate cancer (Review)
- PMID: 40641143
- PMCID: PMC12271840
- DOI: 10.3892/or.2025.8942
Widespread activation and critical role of EMT and stemness in the neuroendocrine differentiation of prostate cancer (Review)
Abstract
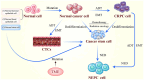
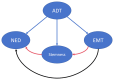
Neuroendocrine (NE) prostate cancer (NEPC) is an aggressive and lethal subtype of prostate cancer. It is typically characterized by the expression of NE markers and the loss of androgen receptor expression. De novo NEPC is rare, accounting for <2% of all prostate cancer cases at diagnosis. More commonly, NEPC arises from prostate adenocarcinoma following androgen deprivation therapy, with 20‑25% of metastatic castration‑resistant prostate cancers undergoing NE differentiation due to lineage plasticity. During this transition, pathways associated with epithelial‑mesenchymal transition (EMT) and stemness are broadly activated, which is considered to be a key driver of NEPC's high metastatic potential, resistance to chemotherapy and radiotherapy and poor prognosis. EMT facilitates metastasis by enhancing cellular motility and invasiveness, while stemness properties contribute to post‑metastatic colonization, immune evasion, therapy resistance and cellular dormancy. As manifestations of cellular plasticity, these processes share overlapping molecular mechanisms. Targeting key regulators within these pathways may offer promising therapeutic strategies for NEPC.
Keywords: epithelial‑mesenchymal transition; molecular mechanism androgen signaling; neuroendocrine prostate cancer; stemness.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Alternative RNA splicing of the GIT1 gene is associated with neuroendocrine prostate cancer.Cancer Sci. 2019 Jan;110(1):245-255. doi: 10.1111/cas.13869. Epub 2018 Dec 12. Cancer Sci. 2019. PMID: 30417466 Free PMC article.
-
Nuclear receptor TLX functions to promote cancer stemness and EMT in prostate cancer via its direct transactivation of CD44 and stem cell-regulatory transcription factors.Br J Cancer. 2024 Nov;131(9):1450-1462. doi: 10.1038/s41416-024-02843-z. Epub 2024 Sep 26. Br J Cancer. 2024. PMID: 39322688
-
[Neuroendocrine differentiation in prostate neoplasms. German version].Pathologie (Heidelb). 2025 Sep;46(5):285-293. doi: 10.1007/s00292-025-01447-5. Epub 2025 Jul 16. Pathologie (Heidelb). 2025. PMID: 40668272 Free PMC article. Review. German.
-
Plexin D1 emerges as a novel target in the development of neural lineage plasticity in treatment-resistant prostate cancer.Oncogene. 2024 Jul;43(30):2325-2337. doi: 10.1038/s41388-024-03081-6. Epub 2024 Jun 14. Oncogene. 2024. PMID: 38877132 Free PMC article.
-
Unlocking the Role of OCT4 in Cancer Lineage Plasticity: A Cross-Cancer Perspective with an Emphasis on Prostate Cancer.Biomedicines. 2025 Jul 4;13(7):1642. doi: 10.3390/biomedicines13071642. Biomedicines. 2025. PMID: 40722714 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

