Impaired Autophagic Flux in Skeletal Muscle of Plectin-Related Epidermolysis Bullosa Simplex With Muscular Dystrophy
- PMID: 40641151
- PMCID: PMC12246382
- DOI: 10.1002/jcsm.70001
Impaired Autophagic Flux in Skeletal Muscle of Plectin-Related Epidermolysis Bullosa Simplex With Muscular Dystrophy
Abstract
Background: Plectin, a multifunctional cytolinker and intermediate filament stabilizing protein, is essential for muscle fibre integrity and function. Mutations in the human plectin gene (PLEC) cause autosomal recessive epidermolysis bullosa simplex with muscular dystrophy (EBS-MD). The disorganization and aggregation of desmin filaments in conjunction with degenerative changes of the myofibrillar apparatus are key features in the skeletal muscle pathology of EBS-MD. We performed a comprehensive analysis addressing protein homeostasis in this rare protein aggregation disease by using human EBS-MD tissue, plectin knock-out mice and plectin-deficient cells.
Methods: Protein degradation pathways were analysed in muscles from EBS-MD patients, muscle-specific conditional plectin knockout (MCK-Cre/cKO) mice, as well as in plectin-deficient (Plec-/-) myoblasts by electron and immunofluorescence microscopy. To obtain a comprehensive picture of autophagic processes, we evaluated the transcriptional regulation and expression levels of autophagic markers in plectin-deficient muscles and myoblasts (RNA-Seq, qRT-PCR, immunoblotting). Autophagic turnover was dynamically assessed by measuring baseline autophagy as well as specific inhibition and activation in mCherry-EGFP-LC3B-expressing Plec+/+ and Plec-/- myoblasts, and by monitoring primary Plec+/+ and Plec-/- myoblasts using organelle-specific dyes. Wild-type and MCK-Cre/cKO mice were treated with chloroquine or metformin to assess the effects of autophagy inhibition and activation in vivo.
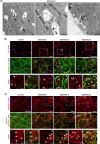
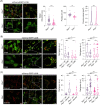
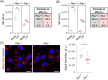
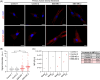
Results: Our study identified the accumulation of degradative vacuoles as well as LC3- and SQSTM1-positive patches in EBS-MD patients, MCK-Cre/cKO mouse muscles and Plec-/- myoblasts. The transcriptional regulation of ~30% of autophagy-related genes was altered, and protein levels of downstream targets of the autophagosomal degradation machinery were elevated in MCK-Cre/cKO muscle lysates (e.g., LAMP2, BAG3 and SQSTM1 to ~160, ~150 and ~140% of controls, respectively; p < 0.05). Autophagosome turnover was compromised in mCherry-EGFP-LC3B-expressing Plec-/- myoblasts (~40% reduction in median red:green ratio, reduced puncta number, smaller puncta; p < 0.01). By labelling autophagic compartments with CYTO-ID dye or lysosomes with LYSO-ID, we found reduced signal intensities in primary Plec-/- cells (p < 0.001). Treatment with chloroquine led to drastic swelling of autophagic vacuoles in primary Plec+/+ myoblasts, while the swelling in Plec-/- cells was moderate, establishing a defect in their autophagic clearance. Chloroquine treatment of MCK-Cre/cKO mice corroborated that loss of plectin coincides with impaired autophagic clearance, while metformin amelioratively induced autophagic flux.
Conclusions: Our work demonstrates that the characteristic protein aggregation pathology in EBS-MD is linked to an impaired autophagic flux. The obtained results open a new perspective on the understanding of the protein aggregation pathology in plectin-related disorders and provide a basis for further pharmacological intervention.
Keywords: autophagy; desmin; epidermolysis bullosa simplex with muscular dystrophy; myofibrillar myopathy; plectin; skeletal muscle.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Missense and Inframe Pathogenic Variants in PLEC Lead to Minimal or Delayed-Onset Muscular Dystrophy in Autosomal Recessive Epidermolysis Bullosa Simplex: A Genotype-Phenotype Correlation in Nine Cases.J Dermatol. 2025 Aug;52(8):1274-1284. doi: 10.1111/1346-8138.17785. Epub 2025 Jun 17. J Dermatol. 2025. PMID: 40525783
-
Downstream effects of plectin mutations in epidermolysis bullosa simplex with muscular dystrophy.Acta Neuropathol Commun. 2016 Apr 27;4(1):44. doi: 10.1186/s40478-016-0314-7. Acta Neuropathol Commun. 2016. PMID: 27121971 Free PMC article.
-
Muscle-Related Plectinopathies.Cells. 2021 Sep 19;10(9):2480. doi: 10.3390/cells10092480. Cells. 2021. PMID: 34572129 Free PMC article. Review.
-
Plectin isoform P1b and P1d deficiencies differentially affect mitochondrial morphology and function in skeletal muscle.Hum Mol Genet. 2015 Aug 15;24(16):4530-44. doi: 10.1093/hmg/ddv184. Epub 2015 May 27. Hum Mol Genet. 2015. PMID: 26019234 Free PMC article.
-
The many faces of plectin and plectinopathies: pathology and mechanisms.Acta Neuropathol. 2013 Jan;125(1):77-93. doi: 10.1007/s00401-012-1026-0. Epub 2012 Aug 3. Acta Neuropathol. 2013. PMID: 22864774 Review.
References
-
- Agnetti G., Herrmann H., and Cohen S., “New Roles for Desmin in the Maintenance of Muscle Homeostasis,” FEBS Journal 289 (2022): 2755–2770. - PubMed
-
- Rezniczek G. A., Abrahamsberg C., Fuchs P., Spazierer D., and Wiche G., “Plectin 5′‐Transcript Diversity: Short Alternative Sequences Determine Stability of Gene Products, Initiation of Translation and Subcellular Localization of Isoforms,” Human Molecular Genetics 12 (2003): 3181–3194. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

