Skeletal Muscle-Specific Deletion of E3 Ligase Asb2 Enhances Muscle Mass and Strength
- PMID: 40641155
- PMCID: PMC12246383
- DOI: 10.1002/jcsm.70007
Skeletal Muscle-Specific Deletion of E3 Ligase Asb2 Enhances Muscle Mass and Strength
Abstract
Background: Maintaining skeletal muscle mass and strength is crucial to prevent sarcopenia during healthy ageing. Ankyrin repeat and suppressor of cytokine signalling box protein 2 (Asb2), an E3 ligase, has been implicated in regulating muscle mass; however, its roles on muscle strength remain unclear, with mixed findings from previous studies. Overexpression of Asb2 decreases muscle mass, whereas its knockdown delays myoblast differentiation and reduces contractile proteins. Given these contradictory findings, we aimed to clarify the role of Asb2 in muscle mass and strength using a skeletal muscle-specific Asb2 knockout (Asb2 MKO) mouse model. Additionally, we investigate the long-term effects of Asb2 on aged muscle, underlying mechanisms on muscle regulation and metabolic effects of Asb2 MKO mice to better understand its role in muscle function and age-related metabolic diseases.
Methods: Asb2 MKO mice were generated using Acta1-Cre recombinase. Body composition was quantified in male and female mice up to 18 months of age. Muscle strength, energy expenditure and glucose metabolism were evaluated using the grip strength test, mitochondrial oxygen consumption measurement, indirect calorimetry and glucose/insulin tolerance tests. Transcriptomic analyses and siRNA studies were performed to elucidate the mechanisms underlying the Asb2 deletion.
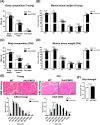
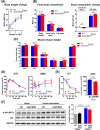
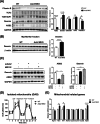
Results: The MKO mice were born healthy and exhibited selective Asb2 deletion in the skeletal muscle, leaving the cardiac muscle unaffected. This deletion led to an increase in the mass of various skeletal muscles (9%-23%, p < 0.05) and improved grip strength (~10%, p < 0.05), both of which were sustained throughout the ageing process. The MKO mice also revealed enhanced mitochondrial function, energy expenditure and whole-body insulin sensitivity. Transcriptomic data supported the muscle phenotype observed in the MKO mice. Notably, desmin, a protein critical for structural integrity and mitochondrial function, was identified as a target protein of the ASB2 E3 ligase.
Conclusions: Skeletal muscle-specific deletion of Asb2 led to increased muscle mass and strength, potentially through preservation of desmin levels. These findings suggest that targeting Asb2 may enhance muscle growth and prevent age-related muscle decline, with potential benefits for metabolic health, particularly by improving mitochondrial function and insulin sensitivity.
Keywords: Asb2; ageing; desmin; mitochondrial function; skeletal muscle mass.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Janssen I., Heymsfield S. B., and Ross R., “Low Relative Skeletal Muscle Mass (Sarcopenia) in Older Persons Is Associated With Functional Impairment and Physical Disability,” Journal of the American Geriatrics Society 50 (2002): 889–896. - PubMed
-
- Landi F., Liperoti R., Russo A., et al., “Sarcopenia as a Risk Factor for Falls in Elderly Individuals: Results From the ilSIRENTE Study,” Clinical Nutrition 31 (2012): 652–658. - PubMed
-
- Guibal F. C., Moog‐Lutz C., Smolewski P., et al., “ASB‐2 Inhibits Growth and Promotes Commitment in Myeloid Leukemia Cells,” Journal of Biological Chemistry 277 (2002): 218–224. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

