Upgrading Ion Migration and Interface Chemistry via a Cyano-Containing COF in a Single-Ion Conductive Polymer toward High-Voltage Lithium-Metal Batteries
- PMID: 40641306
- PMCID: PMC12291442
- DOI: 10.1021/jacs.5c08267
Upgrading Ion Migration and Interface Chemistry via a Cyano-Containing COF in a Single-Ion Conductive Polymer toward High-Voltage Lithium-Metal Batteries
Abstract
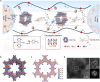
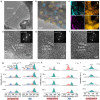
Concentration polarization-triggered dendrite growth hinders the practical application of solid-state polymer lithium batteries, which is caused by the uncontrolled anion migration in conventional dual-ion electrolytes. Single-ion conductive polymer electrolytes (SICPEs) offer a promise to mitigate dendrite growth via reducing concentration polarization and prohibiting salt depletion, yet they are highly challenging for successful implementation due to their narrow electrochemical window and poor ionic conductivity, which result from the deficient dissociation of Li+ polyanions and sluggish chain relaxation. Here, a cyano-containing covalent organic framework (COF) is designed to fuse with SICPEs, promising fast Li+ transport and remarkable interfacial stability toward high-voltage lithium-metal batteries. The electron-withdrawing cyano group on the COF facilitates the dissociation of the polyanions via ion-dipole interactions, resulting in more free-moving Li+. Rapid ion migration then occurs along the long-range cooperative ion transport pathways between the COF and SICPE. Additionally, the cyano group robustly bonds with transition metal ions of NCM cathodes to inhibit the catalytic decomposition of SICPE and guarantee the structural integrity of NCM. Hence, the as-prepared SICPE exhibits a significantly enhanced ionic conductivity of 9.2 × 10-4 S cm-1 and an improved Li+ transference number of 0.94 at room temperature. Accordingly, the NCM622||Li quasi-solid-state cell achieves an exceptional capacity retention of 92.0% over 1000 cycles at 0.5 C, while the cell pairing with the 4.8 V NCM622 cathode delivers a remarkable capacity of 149.5 mAh g-1 after 200 cycles at 0.5 C. This study provides a new perspective for facilitating ionic conductivity and interface chemistry toward the practical feasibility of single-ion conductive polymer electrolytes.
Figures





References
-
- Ning Z., Li G., Melvin D. L. R., Chen Y., Bu J., Spencer-Jolly D., Liu J., Hu B., Gao X., Perera J.. et al. Dendrite initiation and propagation in lithium metal solid-state batteries. Nature. 2023;618(7964):287–293. doi: 10.1038/s41586-023-05970-4. - DOI - PubMed
- Manthiram A., Yu X., Wang S.. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017;2(4):16103. doi: 10.1038/natrevmats.2016.103. - DOI
- Chen R., Li Q., Yu X., Chen L., Li H.. Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem. Rev. 2020;120(14):6820–6877. doi: 10.1021/acs.chemrev.9b00268. - DOI - PubMed
-
- Zhao Q., Liu X., Stalin S., Khan K., Archer L. A.. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy. 2019;4(5):365–373. doi: 10.1038/s41560-019-0349-7. - DOI
- Zou S., Yang Y., Wang J., Zhou X., Wan X., Zhu M., Liu J.. In situ polymerization of solid-state polymer electrolytes for lithium metal batteries: a review. Energy Environ. Sci. 2024;17(13):4426–4460. doi: 10.1039/D4EE00822G. - DOI
- Zhang J., Zhu J., Zhao R., Liu J., Song X., Xu N., Liu Y., Zhang H., Wan X., Ma Y.. et al. An all-in-one free-standing single-ion conducting semi-solid polymer electrolyte for high-performance practical Li metal batteries. Energy Environ. Sci. 2024;17(19):7119–7128. doi: 10.1039/D4EE02208D. - DOI
- Shen J., Chen J., Xu X., Li J., Wang Z., Wang Y., Lin P., Sun J., Huang B., Zhao T.. Engineering d-p orbital hybridization in a single-atom-based solid-state electrolyte for lithium-metal batteries. Angew. Chem., Int. Ed. 2024:e202419367. doi: 10.1002/anie.202419367. - DOI - PubMed
-
- Liu S., Liu W., Ba D., Zhao Y., Ye Y., Li Y., Liu J.. Filler-integrated composite polymer electrolyte for solid-state lithium batteries. Adv. Mater. 2023;35(2):2110423. doi: 10.1002/adma.202110423. - DOI - PubMed
- Liu S., Jiang G., Wang Y., Liu C., Zhang T., Wei Y., An B.. Vitrified metal–organic framework composite electrolyte enabling dendrite-free and long-lifespan solid-state lithium metal batteries. ACS Nano. 2024;18(23):14907–14916. doi: 10.1021/acsnano.3c11725. - DOI - PubMed
- Liu Y., Zou H., Huang Z., Wen Q., Lai J., Zhang Y., Li J., Ding K., Wang J., Lan Y.-Q., Zheng Q.. In situ polymerization of 1,3-dioxane as a highly compatible polymer electrolyte to enable the stable operation of 4.5 V Li-metal batteries. Energy Environ. Sci. 2023;16(12):6110–6119. doi: 10.1039/D3EE02797J. - DOI
- Jiang G., Qu C., Xu F., Zhang E., Lu Q., Cai X., Hausdorf S., Wang H., Kaskel S.. Glassy metal-organic-framework-based quasi-solid-state electrolyte for high-performance lithium-metal batteries. Adv. Funct. Mater. 2021;31(43):2104300. doi: 10.1002/adfm.202104300. - DOI
- Li J., Chen J., Xu X., Wang Z., Shen J., Sun J., Huang B., Zhao T.. Enhanced interphase ion transport via charge-rich space charge layers for ultra-stable solid-state lithium metal batteries. Adv. Energy Mater. 2025;15:2402746. doi: 10.1002/aenm.202402746. - DOI
-
- Huo S., Sheng L., Xue W., Wang L., Xu H., Zhang H., He X.. Challenges of polymer electrolyte with wide electrochemical window for high energy solid-state lithium batteries. InfoMat. 2023;5(3):e12394. doi: 10.1002/inf2.12394. - DOI
- Diederichsen K. M., McShane E. J., McCloskey B. D.. Promising routes to a high Li+ transference number electrolyte for lithium ion batteries. ACS Energy Lett. 2017;2(11):2563–2575. doi: 10.1021/acsenergylett.7b00792. - DOI
-
- Li H., Du Y., Zhang Q., Zhao Y., Lian F.. A single-ion conducting network as rationally coordinating polymer electrolyte for solid-state Li metal batteries. Adv. Energy Mater. 2022;12(13):2103530. doi: 10.1002/aenm.202103530. - DOI
LinkOut - more resources
Full Text Sources

